|
|
 |
| ............................................................. |
|
October 2017 -
Volume 15, Issue 8
|
 |
|
View
this issue in pdf formnat - the issue
has been split into two files for downloading
due to its large size: FULLpdf
(12 MB)
Part
1 &
Part
2
|
|
| ........................................................ |
| From
the Editor |

|
Editorial
A. Abyad (Chief Editor) |
........................................................
|
|
Original Contribution/Clinical Investigation
Immunity
level to diphtheria in beta thalassemia patients
DOI: 10.5742/MEWFM.2017.93048
[pdf
version]
Abdolreza Sotoodeh Jahromi, Karamatollah Rahmanian,
Abdolali Sapidkar, Hassan Zabetian, Alireza
Yusefi, Farshid Kafilzadeh, Mohammad Kargar,
Marzieh Jamalidoust,
Abdolhossein Madani
Genetic
Variants of Toll Like Receptor-4 in Patients
with Premature Coronary Artery Disease, South
of Iran
DOI: 10.5742/MEWFM.2017.93049
[pdf
version]
Saeideh Erfanian, Mohammad Shojaei, Fatemeh
Mehdizadeh, Abdolreza Sotoodeh Jahromi, Abdolhossein
Madani, Mohammad Hojjat-Farsangi
Comparison
of postoperative bleeding in patients undergoing
coronary artery bypass surgery in two groups
taking aspirin and aspirin plus CLS clopidogrel
DOI: 10.5742/MEWFM.2017.93050
[pdf
version]
Ali Pooria, Hassan Teimouri, Mostafa Cheraghi,
Babak Baharvand Ahmadi, Mehrdad Namdari, Reza
Alipoor
Comparison
of lower uterine segment thickness among nulliparous
pregnant women without uterine scar and pregnant
women with previous cesarean section: ultrasound
study
DOI: 10.5742/MEWFM.2017.93051
[pdf version]
Taravat Fakheri, Irandokht Alimohammadi, Nazanin
Farshchian, Maryam Hematti,
Anisodowleh Nankali, Farahnaz Keshavarzi, Soheil
Saeidiborojeni
Effect
of Environmental and Behavioral Interventions
on Physiological and Behavioral Responses of
Premature Neonates Candidates Admitted for Intravenous
Catheter Insertion in Neonatal Intensive Care
Units
DOI: 10.5742/MEWFM.2017.93052
[pdf
version]
Shohreh Taheri, Maryam Marofi, Anahita Masoumpoor,
Malihe Nasiri
Effect
of 8 weeks Rhythmic aerobic exercise on serum
Resistin and body mass index of overweight and
obese women
DOI: 10.5742/MEWFM.2017.93053
[pdf
version]
Khadijeh Molaei, Ahmad Shahdadi, Reza Delavar
Study
of changes in leptin and body mass composition
with overweight and obesity following 8 weeks
of Aerobic exercise
DOI: 10.5742/MEWFM.2017.93054
[pdf
version]
Khadijeh Molaei, Abbas Salehikia
A reassessment
of factor structure of the Short Form Health
Survey (SF-36): A comparative approach
DOI: 10.5742/MEWFM.2017.93088
[pdf version]
Vida Alizad, Manouchehr Azkhosh, Ali Asgari,
Karyn Gonano
Population and Community Studies
Evaluation
of seizures in pregnant women in Kerman - Iran
DOI: 10.5742/MEWFM.2017.93056
[pdf
version]
Hossein Ali Ebrahimi, Elahe Arabpour, Kaveh
Shafeie, Narges Khanjani
Studying
the relation of quality work life with socio-economic
status and general health among the employees
of Tehran University of Medical Sciences (TUMS)
in 2015
DOI: 10.5742/MEWFM.2017.93057
[pdf version]
Hossein Dargahi, Samereh Yaghobian, Seyedeh
Hoda Mousavi, Majid Shekari Darbandi, Soheil
Mokhtari, Mohsen Mohammadi, Seyede Fateme Hosseini
Factors
that encourage early marriage and motherhood
from the perspective of Iranian adolescent mothers:
a qualitative study
DOI: 10.5742/MEWFM.2017.93058
[pdf
version]
Maasoumeh Mangeli, Masoud Rayyani, Mohammad
Ali Cheraghi, Batool Tirgari
The
Effectiveness of Cognitive-Existential Group
Therapy on Reducing Existential Anxiety in the
Elderly
DOI: 10.5742/MEWFM.2017.93059
[pdf
version]
Somayeh Barekati, Bahman Bahmani, Maede Naghiyaaee,
Mahgam Afrasiabi, Roya Marsa
Post-mortem
Distribution of Morphine in Cadavers Body Fluids
DOI: 10.5742/MEWFM.2017.93060
[pdf
version]
Ramin Elmi, Mitra Akbari, Jaber Gharehdaghi,
Ardeshir Sheikhazadi, Saeed Padidar, Shirin
Elmi
Application
of Social Networks to Support Students' Language
Learning Skills in Blended Approach
DOI: 10.5742/MEWFM.2017.93061
[pdf
version]
Fatemeh Jafarkhani, Zahra Jamebozorg, Maryam
Brahman
The
Relationship between Chronic Pain and Obesity:
The Mediating Role of Anxiety
DOI: 10.5742/MEWFM.2017.93062
[pdf
version]
Leila Shateri, Hamid Shamsipour, Zahra Hoshyari,
Elnaz Mousavi, Leila Saleck, Faezeh Ojagh
Implementation
status of moral codes among nurses
DOI: 10.5742/MEWFM.2017.93063
[pdf
version]
Maryam Ban, Hojat Zareh Houshyari Khah, Marzieh
Ghassemi, Sajedeh Mousaviasl, Mohammad Khavasi,
Narjes Asadi, Mohammad Amin Harizavi, Saeedeh
Elhami
The comparison
of quality of life, self-efficacy and resiliency
in infertile and fertile women
DOI: 10.5742/MEWFM.2017.93064
[pdf version]
Mahya Shamsi Sani, Mohammadreza Tamannaeifar
Brain MRI Findings in Children (2-4 years old)
with Autism
DOI: 10.5742/MEWFM.2017.93055
[pdf
version]
Mohammad Hasan Mohammadi, Farah Ashraf Zadeh,
Javad Akhondian, Maryam Hojjati,
Mehdi Momennezhad
Reviews
TECTA gene function and hearing: a review
DOI: 10.5742/MEWFM.2017.93065
[pdf version]
Morteza Hashemzadeh-Chaleshtori, Fahimeh Moradi,
Raziyeh Karami-Eshkaftaki,
Samira Asgharzade
Mandibular
canal & its incisive branch: A CBCT study
DOI: 10.5742/MEWFM.2017.93066
[pdf
version]
Sina Haghanifar, Ehsan Moudi, Ali Bijani, Somayyehsadat
Lavasani, Ahmadreza Lameh
The
role of Astronomy education in daily life
DOI: 10.5742/MEWFM.2017.93067
[pdf
version]
Ashrafoalsadat Shekarbaghani
Human brain
functional connectivity in resting-state fMRI
data across the range of weeks
DOI: 10.5742/MEWFM.2017.93068
[pdf version]
Nasrin Borumandnia, Hamid Alavi Majd, Farid
Zayeri, Ahmad Reza Baghestani,
Mohammad Tabatabaee, Fariborz Faegh
International Health Affairs
A
brief review of the components of national strategies
for suicide prevention suggested by the World
Health Organization
DOI: 10.5742/MEWFM.2017.93069
[pdf
version]
Mohsen Rezaeian
Education and Training
Evaluating
the Process of Recruiting Faculty Members in
Universities and Higher Education and Research
Institutes Affiliated to Ministry of Health
and Medical Education in Iran
DOI: 10.5742/MEWFM.2017.93070
[pdf
version]
Abdolreza Gilavand
Comparison
of spiritual well-being and social health among
the students attending group and individual
religious rites
DOI: 10.5742/MEWFM.2017.93071
[pdf
version]
Masoud Nikfarjam, Saeid Heidari-Soureshjani,
Abolfazl Khoshdel, Parisa Asmand, Forouzan Ganji
A
Comparative Study of Motivation for Major Choices
between Nursing and Midwifery Students at Bushehr
University of Medical Sciences
DOI: 10.5742/MEWFM.2017.93072
[pdf
version]
Farzaneh Norouzi, Shahnaz Pouladi, Razieh Bagherzadeh
Clinical Research and Methods
Barriers
to the management of ventilator-associated pneumonia:
A qualitative study of critical care nurses'
experiences
DOI: 10.5742/MEWFM.2017.93073
[pdf version]
Fereshteh Rashnou, Tahereh Toulabi, Shirin Hasanvand,
Mohammad Javad Tarrahi
Clinical
Risk Index for Neonates II score for the prediction
of mortality risk in premature neonates with
very low birth weight
DOI: 10.5742/MEWFM.2017.93074
[pdf
version]
Azadeh Jafrasteh, Parastoo Baharvand, Fatemeh
Karami
Effect
of pre-colporrhaphic physiotherapy on the outcomes
of women with pelvic organ prolapse
DOI: 10.5742/MEWFM.2017.93075
[pdf
version]
Mahnaz Yavangi, Tahereh Mahmoodvand, Saeid Heidari-Soureshjani
The
effect of Hypertonic Dextrose injection on the
control of pains associated with knee osteoarthritis
DOI: 10.5742/MEWFM.2017.93076
[pdf
version]
Mahshid Ghasemi, Faranak Behnaz, Mohammadreza
Minator Sajjadi, Reza Zandi,
Masoud Hashemi
Evaluation
of Psycho-Social Factors Influential on Emotional
Divorce among Attendants to Social Emergency
Services
DOI: 10.5742/MEWFM.2017.93077
[pdf
version]
Farangis Soltanian
Models and Systems of Health Care
Organizational
Justice and Trust Perceptions: A Comparison
of Nurses in public and private hospitals
DOI: 10.5742/MEWFM.2017.93078
[pdf
version]
Mahboobeh Rajabi, Zahra Esmaeli Abdar, Leila
Agoush
Case series and Case reports
Evaluation
of Blood Levels of Leptin Hormone Before and
After the Treatment with Metformin
DOI: 10.5742/MEWFM.2017.93079
[pdf
version]
Elham Jafarpour
Etiology,
Epidemiologic Characteristics and Clinical Pattern
of Children with Febrile Convulsion Admitted
to Hospitals of Germi and Parsabad towns in
2016
DOI: 10.5742/MEWFM.2017.93080
[pdf
version]
Mehri SeyedJavadi, Roghayeh Naseri, Shohreh
Moshfeghi, Irandokht Allahyari, Vahid Izadi,
Raheleh Mohammadi,
Faculty development
The
comparison of the effect of two different teaching
methods of role-playing and video feedback on
learning Cardiopulmonary Resuscitation (CPR)
DOI: 10.5742/MEWFM.2017.93081
[pdf
version]
Yasamin Hacham Bachari, Leila Fahkarzadeh, Abdol
Ali Shariati
Office based family medicine
Effectiveness
of Group Counseling With Acceptance and Commitment
Therapy Approach on Couples' Marital Adjustment
DOI: 10.5742/MEWFM.2017.93082
[pdf
version]
Arash Ziapour, Fatmeh Mahmoodi, Fatemeh Dehghan,
Seyed Mehdi Hoseini Mehdi Abadi,
Edris Azami, Mohsen Rezaei
|
|
Chief
Editor -
Abdulrazak
Abyad
MD, MPH, MBA, AGSF, AFCHSE
.........................................................
Editorial
Office -
Abyad Medical Center & Middle East Longevity
Institute
Azmi Street, Abdo Center,
PO BOX 618
Tripoli, Lebanon
Phone: (961) 6-443684
Fax: (961) 6-443685
Email:
aabyad@cyberia.net.lb
.........................................................
Publisher
-
Lesley
Pocock
medi+WORLD International
11 Colston Avenue,
Sherbrooke 3789
AUSTRALIA
Phone: +61 (3) 9005 9847
Fax: +61 (3) 9012 5857
Email:
lesleypocock@mediworld.com.au
.........................................................
Editorial
Enquiries -
abyad@cyberia.net.lb
.........................................................
Advertising
Enquiries -
lesleypocock@mediworld.com.au
.........................................................
While all
efforts have been made to ensure the accuracy
of the information in this journal, opinions
expressed are those of the authors and do not
necessarily reflect the views of The Publishers,
Editor or the Editorial Board. The publishers,
Editor and Editorial Board cannot be held responsible
for errors or any consequences arising from
the use of information contained in this journal;
or the views and opinions expressed. Publication
of any advertisements does not constitute any
endorsement by the Publishers and Editors of
the product advertised.
The contents
of this journal are copyright. Apart from any
fair dealing for purposes of private study,
research, criticism or review, as permitted
under the Australian Copyright Act, no part
of this program may be reproduced without the
permission of the publisher.
|
|
|
| October 2017 -
Volume 15, Issue 8 |
|
|
TECTA gene function and
hearing loss: a review
Morteza Hashemzadeh-Chaleshtori,
Fahimeh Moradi,
Raziyeh Karami-Eshkaftaki,
Samira Asgharzade
Cellular
and Molecular Research Center, Basic Health
Sciences Institute, Shahrekord University of
Medical Sciences, Shahrekord, Iran
Correspondence:
Samira Asgharzade
Cellular and Molecular Research Center,
Basic Health Sciences Institute,
Shahrekord University of Medical Sciences,
Shahrekord, Iran
Email: Asgharzade2336@gmail.com
|
Abstract
Hearing loss is considered as the most
prevalent impairment worldwide. It is
one of the most genetically heterogeneous,
which makes molecular diagnosis challenging
in most cases. TECTA is a modular, non-collagenous
protein of the tectorial membrane that
plays a more dynamic role in normal hearing.
Mutation in TECTA cause dominant and recessive
forms of non-syndromic hearing loss. The
clinical findings suggest stable, moderate-to-severe
forms of hereditary hearing loss may be
diagnostic of a mutation in TECTA. In
this review, Directory of Open Access
Journals (DOAJ), Pub Med, Google Scholar
LISTA (EBSCO), Embase, and Web of Science
were searched using relevant search terms
to retrieve eligible publications. This
paper provides an overview of (1) TECTA
gene function, (2) the prevalence of TECTA
related hearing loss, disease symptoms,
(3) identification pattern and (4) animal
models. It also summarizes how mutations
in TECTA induced hearing loss with mid-frequency
audio profile pattern.
Key words:
Hearing loss, Mutation, TECTA gene
|
Sensory and neurological diseases are one of
the largest medical complex problems and (1,
2), hearing loss is the most common neural sensory
disorder in human (3, 4). In developing countries
one out of 500 neonates are born deaf (5). In
50-60 percent of patients the cause of the disease
is deterioration in the function of a single
gene (3). 70% of all hereditary hearing loss
is non-syndromic and 30% is syndromic (6). Non-syndromic
hearing impairment is extremely heterogeneous;
68 autosomal recessive loci (DFNB), 52 autosomal
dominant loci (DFNA), 5 involved loci on X chromosome
and 2 involved loci on Y chromosome has been
reported so far (7). Hearing loss caused by
TECTA mutations are inherited in two forms of
autosomal dominant (DFNA8/12MIM 601543-MIM601842)
and autosomal recessive (DFNB21). Mutation in
the TECTA gene is the cause of 4% of all non-syndromic
autosomal dominant hearing loss and has been
reported in various kinds of hearing impairments
in different populations (8). The most mutations
related to DFNB21 have been found in Iran (9).
Patients ‘audiometric pattern’ is
flat or U shaped in the mild or mild to severe
frequencies (10). Patients’ audiograms
are considered as the most important tools to
identify mutations in the TECTA gene (10). In
this review article, we aimed at investigating
TECTA gene function; the prevalence of TECTA
related hearing loss, disease symptoms, identification
patterns and related animal models.
Scientific databases Directory of Open Access
Journals (DOAJ), Google Scholar, Pub Med, LISTA
(EBSCO), Embase, and Web of Science were searched
using relevant search terms to retrieve eligible
publications on structure and function, audiometric
pattern and inheritance pattern of hearing loss
and animal modeling related to the TECTA gene.
TECTA gene structure and function
Human TECTA gene (MIM 602574, Gene ID 7007)
has been located at 11q22–q24 and mouse
TECTA gene is on chromosome 9 (8). Studies have
revealed that TECTA is highly conserved in zebrafish,
mice and humans (11). Alpha tectorin is encoded
by the TECTA gene and is one of the most important
non-collagen parts of the tectorial membrane
of the inner ear (12). The TECTA gene contains
23 exons and renders a protein of 2155 amino
acids (13). Tectorial membrane is a fiber extended
to extracellular matrix and is connected to
stereocilia clusters of sensory hair cells (Figure
1). Sounds cause the movement of tectorial membrane
related cells (14). Stereocilia motions give
rise to the transforming of sound waves into
neural pulse. Tectorial membrane is highly expressed
in the inner ear and is found in three forms
of collagenic (alpha-tectorin), non-collagenic
(beta-tectorin) and glycoprotein (otogelin).
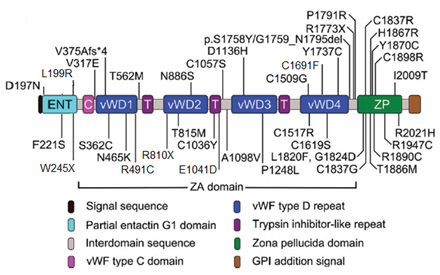
Alpha-tectorin is a large glycoprotein containing
several domains including Entactin G1 (ENT)
domain, the large area of Zonadhesin (ZA) which
includes three factors of von Willebrand factor
type C or D (vWFD V1, V2, V3, V4), N-terminal
entactin G1-like domain and C-terminal Zona
Pellucida and also three trypsins inhibiting
cysteine-rich domains (Figure 2) (12, 14). These
domains have formed a network by disulfide bonds
and in association with beta-tectorin have established
the non-collagenic matrix in tectorial membrane
(11).
Figure 1: Organ of Corti structure and TECTA.
The structure of the organ of corti Schematic
picture in the basal area of the cochlea in
the human ear. TM is connected to the outer
hair-cells Stereocilia via Kimura membrane,
hair-cell Stereociliavia Hensen fibers and also
spiral limbus (11).
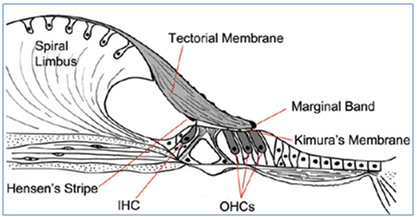
Figure 2: The structures of TECTA domains
and the position of missense mutations causing
hearing loss. Mutation in Entactin-G1 domain,
vWFD, vWFD2 and TIL2 repeats of ZA and ZP cause
to mild-frequency hearing loss, while mutation
in other parts of ZA domain results in high-frequency
hearing impairment.
Audiometric pattern in hearing loss associated
with TECTA deficiency
A large number of mutations associated with
hearing loss have been reported so far. Using
audiogram pattern is an appropriate step to
select the presumably mutated genes. To reduce
costs and save time, surveying audio profile
of the deafness families is an effective step
to screen families for linkage analysis. Studies
have revealed that any mutation in TECTA gene
which inactivates the gene products is associated
with non-syndromic autosomal recessive hearing
loss. Autosomal recessive mutations in TECTA
gene lead to a moderate to severe deafness and
display an audiogram pattern in a flat or U
shape at all frequencies. Fortunately, this
pattern helps to identify TECTA gene as the
cause of some kinds of hearing loss. While all
missense mutations in TECTA gene cause autosomal
dominant type of hearing loss, depending on
the involved domain harboring the mutation,
clinical manifestations are different (10).
Mutations in the ZP domain cause a dominant
negative phenotype giving rise to a disrupted
connection between different tectorin polypeptides,
so deteriorate tectorial membrane structure.
Any defect in this membrane results in a reduction
in the quality of sounds transferred to stereociliary
fibers of hair cells and eventually cause hearing
loss (15). Another hypothesis explains that
any instability of alpha tectorin mRNA or its
destruction lead to decreased protein levels
in tectorial membrane (7). Mutation in ZP domain
causes non-progressive prelingual deafness at
mild frequency, while any mutation in ZA domain
results in progressive hearing loss at the high
frequency range in childhood (16). Researchers
have demonstrated that there is a significant
relationship between mutations in ZP and mild
hearing loss and also ZA and Progressive high
frequency hearing impairment (17). Furthermore,
mutation in Entactin-G1-like domain at the first
repeat of vWFD and also at TIL2 repeats in ZP
and ZA domain cause high-frequency hearing loss.
Even the site of the mutation can affect hearing
loss stability, so missense mutations occurring
at cysteine repeats of ZA and ZP domain cause
progressive post lingual hearing loss (18).
These mutations decay disulfide bonds and destabilize
the cellular matrix structure, while the rest
of the mutations occurring at the other amino
acids in this region cause stable hearing loss.
Deafness related to TECTA involved loci
Non syndromic autosomal recessive hearing
loss associated with DFNB21
The first time in 1999, DFNB21 has been reported
in a Lebanese family with Severe-to-Profound
prelingual deafness by Mustapha et al. This
mutation has been located at the donor splice
site in intron 9 and results in a stop codon
at 972 positions rendering a truncated protein.
This variant has not been observed in 101 healthy
subjects (19). In 2003, in two Iranian and Pakistani
families with Severe-to-Profound sensory neural
hearing loss, respectively an insertion mutation
(649insC (602574.0006)) and a deletion mutation
(6037delG (602574.0007)) have been reported
(20). In 2007, linkage analysis using D11S1299,
D11S1998 and D11S4464 markers surveying 45 GJB2
negative deaf families displayed linkage to
the TECTA gene. Sequencing of the TECTA gene
revealed a frame shift mutation (266delT, p.122X),
a missense mutation (5211C>A, p.Y1737X) and
a 9.6kb deletion in exon 10 and intron 8 and
9 (10). One year later, a 16bp deletion in exon
21 in the Iran population was reported (21).
In 2012, the first compound heterozygote from
a Korean population was reported using next
generation sequencing approach. This missense
mutation has been located in exon 15 and insertion
has occurred at the donor splice site. The father
and mother of this family were heterozygotes
for a missense mutation and a splice site mutation
respectively. Moreover, these mutations have
not been observed in 120 healthy people (17).
In 2016, surveying 50 Iranian families with
Arab ethnicity, the last identified mutation
in the TECTA gene was reported (22). This nonsense
mutation lead to translation of a truncated
protein containing 245 amino acids and was not
observed in healthy volunteers (22) (Table 1).
Click here for Table
1: Reported mutations in TECTA gene (DFNB21)
and their audiogram pattern
Click here for Table
2: reported mutations in TECTA gene (DFNA8/12)
and their audiogram pattern
Autosomal dominant non-syndromic hearing
loss
A study accomplished in 1998 for the first time
reported that two Australian and Belgian families
displayed linkage to DFNB8 and DFNB12 loci at
the long arm of chromosome 9, where the TECTA
gene has been located (7). A compound heterozygous
missense mutation (c.5725C>T and c.5738G>A)
in the distance between 12bp located at exon
17 in a Belgium pedigree was reported in 18
patients while 40 healthy controls lacked the
mutation. c.5876A>G mutation in exon 18 was
reported in an Australian family while the mutation
was not observed in 100 Australian and Belgian
healthy people. These three mutations have been
located in the ZP domain and cause prelingual
hearing loss (7). In 1999 investigating a French
pedigree of mild, moderate and progressive hearing
loss showed linkage disequilibrium to DFNA12.
TECTA gene sequencing revealed a missense mutation
(c.4857G>C) changing cysteine 1916 into serine
(C1916S) giving rise to the removing of cysteine
in CGLC motif of D4vWfin zonadhesin/vonWillebrand
domain (23). CGLC motifs in D1 and D2 repeats
catalyzes the polymerization of disolphide bonds
in VWF and are involved in the formation of
non-collagenic tectorial membrane matrix. This
mutation changes the properties of sound mechanical
transfer in tectorial membrane via disturbing
the proper polypeptide cross-linking, resulting
in hearing loss in patients (23).
Parallel to this study, C1057S mutation in
one domain of zonadhesin/Von Willebrand was
reported in a population of Sweden. C1057S mutation
attenuates sound transmission by changing polypeptide
cross linking, resulting in deafness (8). In
2001 and 2002 two missense mutations in exon
17 and 20 were reported in the Spanish (24)
and Japanese (8) pedigrees respectively and
from 2004-2013 in the USA (10), Turkey (18),
Germany (25), Korea (26) and China, some mutations
were reported which have been described in detail
in Table 2.
The biggest cohort study focusing on DFNA8/12
was accomplished in 2011. In this study 835
American deaf families (autosomal dominant non-syndromic
hearing loss) were investigated. According to
audiometric data, 73 pedigrees that had deafness
at low and high frequency were selected. Their
audiograms were screened by Audio Gene software
(http://audiogene.eng.uiowa.edu/)
which contains a databank including 1926 audiograms
from 17 known loci involved in ADNSHL. Based
on the audiogram pattern, the software predicts
which locus is involved in hearing loss (26,
27). In the next phase of the study, 372 Spanish
deaf were surveyed. Audio gene prediction introduced
64 families with possibility of DFNA8/12 involvement
that TECTA gene sequencing indicated that only
9 families carried the mutation, also 14 mutations
were reported in the Spanish population (26).
In 2014, in China a 9bp deletion was reported
(28). In Table 2, all of the autosomal dominant
mutations have been represented in detail.
|
MOUSE
MODELS
FOR
HUMAN
HEARING
IMPAIRMENT |
TectaENT/ENT
mouse
models
have
been
developed
by
Exon
Skipping,
so
96
amino
acids
were
removed
from
N-terminal
of
entactin
G1
in
alpha-tectorine.
During
the
first
days
of
the
embryonic
period,
examining
the
mouse
model
demonstrated
that
the
greater
epithelial
ridge
of
TECTA
was
very
little
growth
and
also
was
not
detectable
by
western
blot
analysis.
Even
three
weeks
after
the
birth
TECTA
expression
was
negligible,
while
tectorial
membrane
in
Tecta+/+
and
Tecta+/DENT
mice
was
normal
and
TM
had
been
connected
to
Spiral
limbus
fully.
But
in
the
mouse
model,
TM
had
been
separated
from
spiral
limbus
and
the
organ
of
corti
and
also
had
no
beta
tectorial
membrane
and
otogelin
as
the
collagenic
part
of
tectorial
membrane
(29).
Otoconia
membrane
has
been
reduced
in
the
models
compared
to
heterozygotes
or
normal
group.
The
mouse
model
was
not
able
to
do
rotational
movements
and
also
there
were
explicit
defects
in
their
movements
and
behavior.
In
these
mice,
there
were
not
any
appropriate
matrix
filaments
and
sheets,
but
outer
and
inner
hair
cells
were
normal
and
had
positioned
at
the
right
place.
The
results
indicated
that
mutated
alpha
tectorin
protein
is
produced
and
secreted
in
these
mice
but
is
not
able
to
organize
the
matrix
and
is
ruined
rapidly
(29).
The
next
mouse
model
was
the
mice
with
mutation
in
TectaY1870C.
In
ZP
domain,
this
mutation
was
reported
in
1998
in
an
Australian
family
with
prelingual
hair
impairment
at
moderate
to
severe
frequency.
In
these
transgenic
mice,
TM
matrix
structure
was
disturbed
and
ZA
domain
thickness
was
decreased,
although
these
changes
had
no
major
effect
on
the
main
role
of
tectorial
membrane
according
to
the
data
obtained
from
the
evaluating
of
sensitivity
and
frequency
of
cochlea
mechanical
response
to
sounds.
The
nervous
threshold
was
evaluated;
nervous
regulation
was
extended
resulting
in
a
major
decrease
in
the
peak
of
nervous
regulation
curve
(30).
TectaC1509G/+
mouse
model
harbored
a
missense
mutation
in
the
ZA
domain
which
had
caused
a
progressive
mild
to
moderate
hearing
impairment
in
a
Turkish
family.
Structural
phenotype
is
more
subtle,
hearing
response
threshold
of
brain
stem
at
-40
frequencies
was
25dB
throughout
the
hearing
range
and
hearing
loss
occurs
mostly
at
mild
level
(10-35
KHz)
(31).
In
a
study
published
in
2014,
a
three
mouse
model
including
TectaL1820F,
G1824D/+
in
ZP
domain
which
had
caused
deafness
at
mild
frequency
in
a
Belgium
family,
TectaC1837G/+
in
ZA
domain
and
which
had
caused
progressive
hearing
loss
at
mild
frequency
in
a
Spanish
family
and
TectaC1619S/+
in
ZA
domain
which
had
caused
progressive
hearing
loss
at
high
frequency
in
a
French
family,
were
investigated.
Mutations
in
ZA
and
ZP
domain
give
rise
to
distinct
and
different
changes
in
TM
structure
(28).
Changes
in
TM
is
similar
to
the
changes
when
TectaY1870C
mutation
occurs
and
includes
reduction
in
limbus
region,
the
lack
of
striated
sheet
matrix,
disturbance
in
the
organization
of
collagenic
fiber
in
the
Sulcal
region
and
finally
the
lamination
of
Kimura
membrane.
Defects
in
tectorial
membrane
in
TectaC1619S/+
mouse
model
is
completely
different
from
models
harboring
mutations
in
ZP
domain
and
is
similar
to
TectaC1509G/+
mouse
model.
These
defects
include
destroying
marginal
band;
a
major
reduction
in
Covernet
(upper
layer
of
TM
is
covered
by
this
fiber
canal)
and
finally
changes
in
fiber
network
profile
give
rise
to
the
reduction
in
hair
cells
connection
(11).
In
the
case
of
mutations
in
ZP
domain,
the
threshold
of
brain
stem
hearing
response
(in
the
range
of
8-40
KHz)
increased
by
30-40
dB,
while
mice
carrying
mutations
in
ZA
domain
displayed
a
20-30
dB
increase,
although
TM
phenotype
is
stable
and
there
is
no
evidence
implying
gradual
deterioration
of
hearing
structure
or
function
(11).
Regarding
the
data
obtained
from
these
five
DFNA8/12
related
mouse
models,
genotype-phenotype
correlation
related
to
ZP
and
ZA
domain
can
be
clearly
observed,
so
this
clue
can
be
used
in
the
prediction
of
the
involved
domains
in
hearing
impairment
according
to
the
hearing
phenotype.
Acknowledgments
The
authors
would
like
to
acknowledge
Research
and
Technology
Deputy
of
Shahrekord
University
of
Medical
Sciences
for
supporting
this
study.
1.
Asgharzade
S,
Rabiei
Z,
Rafieian-Kopaei
M.
Effects
of
Matricaria
chamomilla
extract
on
motor
coordination
impairment
induced
by
scopolamine
in
rats.
Asian
Pacific
Journal
of
Tropical
Biomedicine.
2015;5(10):829-33.
2.
Rabiei
Z,
Asgharzade
S,
Bigdeli
M.
Medicinal
herbs
effective
in
the
treatment
of
the
Alzheimer’s
disease.
Journal
of
Babol
University
of
Medical
Sciences.
2015;16(15):51-9.
3.
Hashemzadeh
Chaleshtori
M,
Simpson
MA,
Farrokhi
E,
Dolati
M,
Hoghooghi
Rad
L,
Amani
Geshnigani
S,
et
al.
Novel
mutations
in
the
pejvakin
gene
are
associated
with
autosomal
recessive
non-syndromic
hearing
loss
in
Iranian
families.
Clinical
genetics.
2007;72(3):261-3.
4.
Asgharzade
S,
Reiisi
S,
Tabatabaiefar
MA,
Chaleshtori
MH.
Screening
of
Myo7A
Mutations
in
Iranian
Patients
with
Autosomal
Recessive
Hearing
Loss
from
West
of
Iran.
Iranian
journal
of
public
health.
2017;46(1):76.
5.
ASGHARZADE
S,
CHALESHTORI
MH,
Tabatabifar
M,
Reisi
S,
Modaressi
MH.
MUTATION
IN
SECOND
EXON
OF
MYO15A
GENE
CAUSE
OF
NONSYNDROMIC
HEARING
LOSS
AND
ITS
ASSOCIATION
IN
THE
ARAB
POPULATION
IN
IRAN.
Genetika.
2016;48(2):587-96.
6.
Sadeghi
A,
Sanati
MH,
Alasti
F,
Hashemzadeh
Chaleshtori
M,
Ataei
M.
Mutation
analysis
of
connexin
26
gene
and
del
(GJB6-D13S1830)
in
patients
with
hereditary
deafness
from
two
provinces
in
Iran.
Iran
J
Biotechnol.
2005;3(4):255-8.
7.
Verhoeven
K,
Van
Laer
L,
Kirschhofer
K,
Legan
PK,
Hughes
DC,
Schatteman
I,
et
al.
Mutations
in
the
human
-tectorin
gene
cause
autosomal
dominant
non-syndromic
hearing
impairment.
Nature
genetics.
1998;19(1):60-2.
8.
Balciuniene
J,
Dahl
N,
Jalonen
P,
Verhoeven
K,
Van
Camp
G,
Borg
E,
et
al.
Alpha-tectorin
involvement
in
hearing
disabilities:
one
gene-two
phenotypes.
Human
genetics.
1999;105(3):211-6.
9.
Khosrofar
M,
Pourreza
MR,
Asgharzadeh
S,
Tahmasebi
P,
Ali
Asgari
E,
Ghasemikhah
R,
et
al.
Genetic
Linkage
Analysis
of
the
DFNB21
Locus
in
Autosomal
Recessive
Hearing
Loss
in
large
families
from
Khuzestan
Province.
Arak
Medical
University
Journal.
2017;20(3):31-8.
10.
Meyer
NC,
Alasti
F,
Nishimura
CJ,
Imanirad
P,
Kahrizi
K,
Riazalhosseini
Y,
et
al.
Identification
of
three
novel
TECTA
mutations
in
Iranian
families
with
autosomal
recessive
nonsyndromic
hearing
impairment
at
the
DFNB21
locus.
American
Journal
of
Medical
Genetics
Part
A.
2007;143(14):1623-9.
11.
Legan
PK,
Goodyear
RJ,
Morín
M,
Mencia
A,
Pollard
H,
Olavarrieta
L,
et
al.
Three
deaf
mice:
mouse
models
for
TECTA-based
human
hereditary
deafness
reveal
domain-specific
structural
phenotypes
in
the
tectorial
membrane.
Human
molecular
genetics.
2013;23(10):2551-68.
12.
Maeda
Y,
Fukushima
K,
Kasai
N,
Maeta
M,
Nishizaki
K.
Quantification
of
TECTA
and
DFNA5
expression
in
the
developing
mouse
cochlea.
Neuroreport.
2001;12(15):3223-6.
13.
Richardson
G,
Forge
A,
Kros
C,
Fleming
J,
Brown
S,
Steel
K.
Myosin
VIIA
is
required
for
aminoglycoside
accumulation
in
cochlear
hair
cells.
Journal
of
Neuroscience.
1997;17(24):9506-19.
14.
Richardson
GP,
de
Monvel
JB,
Petit
C.
How
the
genetics
of
deafness
illuminates
auditory
physiology.
Annual
review
of
physiology.
2011;73:311-34.
15.
Hashemzadeh-Chaleshtori
M,
Saidijam
M,
Jami
M-S,
Ghasemi-Dehkordi
P.
MicroRNA-183
Family
in
Inner
Ear:
Hair
Cell
Development
and
Deafness.
Journal
of
audiology
&
otology.
2016;20(3):131-8.
16.
Plantinga
RF,
de
Brouwer
AP,
Huygen
PL,
Kunst
HP,
Kremer
H,
Cremers
CW.
A
novel
TECTA
mutation
in
a
Dutch
DFNA8/12
family
confirms
genotype–phenotype
correlation.
Journal
of
the
Association
for
Research
in
Otolaryngology.
2006;7(2):173-81.
17.
Sagong
B,
Baek
JI,
Bok
J,
Lee
KY,
Kim
UK.
Identification
of
a
nonsense
mutation
in
the
STRC
gene
in
a
Korean
family
with
moderate
hearing
loss.
Int
J
Pediatr
Otorhinolaryngol.
2016;80:78-81.
18.
Pfister
M,
Thiele
H,
Van
Camp
G,
Fransen
E,
Apaydin
F,
Aydin
Ö,
et
al.
A
genotype-phenotype
correlation
with
gender-effect
for
hearing
impairment
caused
by
TECTA
mutations.
Cellular
Physiology
and
Biochemistry.
2004;14(4-6):369-76.
19.
Mustapha
M,
Weil
D,
Chardenoux
S,
Elias
S,
El-Zir
E,
Beckmann
JS,
et
al.
An
-tectorin
gene
defect
causes
a
newly
identified
autosomal
recessive
form
of
sensorineural
pre-lingual
non-syndromic
deafness,
DFNB21.
Human
molecular
genetics.
1999;8(3):409-12.
20.
Naz
S,
Alasti
F,
Mowjoodi
A,
Riazuddin
S,
Sanati
M,
Friedman
T,
et
al.
Distinctive
audiometric
profile
associated
with
DFNB21
alleles
of
TECTA.
Journal
of
medical
genetics.
2003;40(5):360-3.
21.
Alasti
F,
Sanati
MH,
Behrouzifard
AH,
Sadeghi
A,
De
Brouwer
AP,
Kremer
H,
et
al.
A
novel
TECTA
mutation
confirms
the
recognizable
phenotype
among
autosomal
recessive
hearing
impairment
families.
International
journal
of
pediatric
otorhinolaryngology.
2008;72(2):249-55.
22.
Asgharzade
S,
Tabatabaiefar
MA,
Modarressi
MH,
Ghahremani
MH,
Reiisi
S,
Tahmasebi
P,
et
al.
A
novel
TECTA
mutation
causes
ARNSHL.
International
Journal
of
Pediatric
Otorhinolaryngology.
2017;92:88-93.
23.
Alloisio
N,
Morle
L,
Bozon
M,
Godet
J,
Verhoeven
K,
Van
Camp
G,
et
al.
Mutation
in
the
zonadhesin-like
domain
of
-tectorin
associated
with
autosomal
dominant
non-syndromic
hearing
loss.
European
Journal
of
Human
Genetics.
1999;7(2):255-8.
24.
Moreno-Pelayo
MA,
del
Castillo
I,
Villamar
M,
Romero
L,
Hernández-Calvín
FJ,
Herraiz
C,
et
al.
A
cysteine
substitution
in
the
zona
pellucida
domain
of
-tectorin
results
in
autosomal
dominant,
postlingual,
progressive,
mid
frequency
hearing
loss
in
a
Spanish
family.
Journal
of
medical
genetics.
2001;38(5):e13-e.
25.
Collin
RW,
de
Heer
A-MR,
Oostrik
J,
Pauw
R-J,
Plantinga
RF,
Huygen
PL,
et
al.
Mid-frequency
DFNA8/12
hearing
loss
caused
by
a
synonymous
TECTA
mutation
that
affects
an
exonic
splice
enhancer.
European
journal
of
human
genetics.
2008;16(12).
26.
Hildebrand
MS,
Morín
M,
Meyer
NC,
Mayo
F,
Modamio-Hoybjor
S,
Mencía
A,
et
al.
DFNA8/12
caused
by
TECTA
mutations
is
the
most
identified
subtype
of
nonsyndromic
autosomal
dominant
hearing
loss.
Human
mutation.
2011;32(7):825-34.
26.
Hildebrand
MS,
Morín
M,
Meyer
NC,
Mayo
F,
Modamio-Hoybjor
S,
Mencía
A,
et
al.
DFNA8/12
caused
by
TECTA
mutations
is
the
most
identified
subtype
of
nonsyndromic
autosomal
dominant
hearing
loss.
Human
mutation.
2011;32(7):825-34.
27.
Hildebrand
MS,
Tack
D,
McMordie
SJ,
Deluca
A,
Hur
IA,
Nishimura
C,
et
al.
Audioprofile-directed
screening
identifies
novel
mutations
in
KCNQ4
causing
hearing
loss
at
the
DFNA2
locus.
Genetics
in
Medicine.
2008;10(11):797-804.
28.
Su
Y,
Tang
W-X,
Gao
X,
Yu
F,
Dai
Z-Y,
Zhao
J-D,
et
al.
A
novel
mutation
in
the
TECTA
gene
in
a
Chinese
family
with
autosomal
dominant
nonsyndromic
hearing
loss.
PloS
one.
2014;9(2):e89240.
29.
Legan
PK,
Lukashkina
VA,
Goodyear
RJ,
Kössl
M,
Russell
IJ,
Richardson
GP.
A
targeted
deletion
in
-tectorin
reveals
that
the
tectorial
membrane
is
required
for
the
gain
and
timing
of
cochlear
feedback.
Neuron.
2000;28(1):273-85.
30.
Legan
PK,
Lukashkina
VA,
Goodyear
RJ,
Lukashkin
AN,
Verhoeven
K,
Van
Camp
G,
et
al.
A
deafness
mutation
isolates
a
second
role
for
the
tectorial
membrane
in
hearing.
Nature
neuroscience.
2005;8(8):1035-42.
31.
Xia
A,
Gao
SS,
Yuan
T,
Osborn
A,
Bress
A,
Pfister
M,
et
al.
Deficient
forward
transduction
and
enhanced
reverse
transduction
in
the
alpha
tectorin
C1509G
human
hearing
loss
mutation.
Disease
models
&
mechanisms.
2010;3(3-4):209-23.
32.
Miyagawa
M,
Nishio
SY,
Hattori
M,
Moteki
H,
Kobayashi
Y,
Sato
H,
et
al.
Mutations
in
the
MYO15A
gene
are
a
significant
cause
of
nonsyndromic
hearing
loss:
massively
parallel
DNA
sequencing-based
analysis.
Ann
Otol
Rhinol
Laryngol.
2015;124
Suppl
1:158S-68S.
33.
Hutchin
T,
Coy
N,
Conlon
H,
Telford
E,
Bromelow
K,
Blaydon
D,
et
al.
Assessment
of
the
genetic
causes
of
recessive
childhood
non-syndromic
deafness
in
the
UK–implications
for
genetic
testing.
Clinical
genetics.
2005;68(6):506-12.
34.
King
DA,
Fitzgerald
TW,
Miller
R,
Canham
N,
Clayton-Smith
J,
Johnson
D,
et
al.
A
novel
method
for
detecting
uniparental
disomy
from
trio
genotypes
identifies
a
significant
excess
in
children
with
developmental
disorders.
Genome
research.
2014;24(4):673-87.
35.
Li
Z,
Guo
Y,
Lu
Y,
Li
J,
Jin
Z,
Li
H,
et
al.
Identification
of
a
novel
TECTA
mutation
in
a
Chinese
DFNA8/12
family
with
prelingual
progressive
sensorineural
hearing
impairment.
PloS
one.
2013;8(7):e70134.
36.
Behlouli
A,
Bonnet
C,
Abdi
S,
Hasbellaoui
M,
Boudjenah
F,
Hardelin
J-P,
et
al.
A
novel
biallelic
splice
site
mutation
of
TECTA
causes
moderate
to
severe
hearing
impairment
in
an
Algerian
family.
International
Journal
of
Pediatric
Otorhinolaryngology.
2016;87:28-33.
37.
Diaz-Horta
O,
Duman
D,
Foster
II
J,
Srmac
A,
Gonzalez
M,
Mahdieh
N,
et
al.
Whole-exome
sequencing
efficiently
detects
rare
mutations
in
autosomal
recessive
nonsyndromic
hearing
loss.
PloS
one.
2012;7(11):e50628.
38.
Alford
RL.
Nonsyndromic
hereditary
hearing
loss.
Medical
Genetics
in
the
Clinical
Practice
of
ORL.
70:
Karger
Publishers;
2011.
p.
37-42.
39.
Iwasaki
S,
Harada
D,
Usami
S-i,
Nagura
M,
Takeshita
T,
Hoshino
T.
Association
of
clinical
features
with
mutation
of
TECTA
in
a
family
with
autosomal
dominant
hearing
loss.
Archives
of
Otolaryngology–Head
&
Neck
Surgery.
2002;128(8):913-7.
|
|
.................................................................................................................

|
| |
|

