|
The effect of Hypertonic
Dextrose injection on the control of pain associated
with knee osteoarthritis
Mahshid Ghasemi (1)
Faranak Behnaz (2)
Mohammadreza Minator Sajjadi (3)
Reza Zandi (4)
Masoud Hashemi (5)
(1) Assistant Professor of Anesthesiology,
Shahid Beheshti University of Medical Sciences,
Tehran, Iran
(2) Assistant Professor of Anesthesiology, Shohada
hospital, Shahid Beheshti University, Tehran,
Iran
(3) Orthopaedic surgeon, Taleghani Hospital
Research Development unit, Saheed Beheshti University
of medical Sciences, Tehran, Iran
(4) M.D .Assistant Professor of Orthopedic Department,
Shahid Beheshti University of medical sciences,
Taleghani hospital, Tehran, Iran
(5) Associate Professor of Anesthesiology, Shahid
Beheshti University of Medical Sciences, Akhtar
hospital, Tehran, Iran
Correspondence:
Masoud Hashemi
Associate Professor of Anesthesiology, Shahid
Beheshti University of Medical Sciences, Akhtar
hospital, Tehran, Iran
Email: dr.hashemi@sbmu.ac.ir
|
Abstract
Introduction: The
purpose of this study was to evaluate
the effect of dextrose injection on controlling
pain associated with knee osteoarthritis.
Methods: To
achieve the research objectives, available
sampling was done using 80 patients with
knee osteoarthritis referring to Taleghani
Hospital in 2017 and samples were divided
into two groups: 15% dextrose injection
and 25% hypertonic dextrose injection.
This injection was performed at the beginning
of the study, the first week, the fifth
week and the ninth week. During these
weeks, participants were asked to complete
the WOMAC questionnaire implementing the
VAS scale. After data collection, independent
t-test and two-way variance analysis with
repeated measures were used.
Findings: The findings showed that
15% and 25% dextrose injection had a significant
effect on the visual scale of pain and
function of patients, so that, during
weekly treatment, scales showed improvement
in treatment in these patients. Also,
other findings showed that injection of
25% dextrose had a significant visual
analog of patient’s pain and function
compared to 15%.
Conclusion:
In general, it can be suggested that the
use of dextrose prolotherapy is a simple,
safe, inexpensive, accessible and less
complicated method than other treatments
in these patients.
Key words:
Osteoarthritis, Prolotherapy, Treatment,
Health.
|
Osteoarthritis (OA) is the most common joint
disease in humans and is characterized by the
degradation of the hyaline cartilage and can
lead to chronic pain and severe disability in
the patient (1). The morning and the decrease
in the movement range of the joint are important
characteristics of this disease (2). The greatest
risk factor for this disease is age (3), but
high blood pressure, severe strokes, excessive
use of the joint, inoperative anterior cruciate
ligament and damage to the meniscus can also
result in knee OA (4-5). OA levels in all societies
are rising due to increased longevity. Pain,
stiffness and knee pain during active knee movements
are common symptoms of OA, which not only reduces
the ability of patients, but also adversely
affects the quality of life of patients (6).
Osteoarthritis is one of the five main causes
of physical disability in the elderly (1, 7).
It is estimated that 90% of people over 40 in
the United States suffer from osteoarthritis
(8). Studies show that the prevalence of knee
osteoarthritis is 60 to 90% as a cause of musculoskeletal
pain among people 65 years of age or older (9).
By 2020, it is estimated approximately 4.55
million Americans, i.e. 18.2% of the US population
will have osteoarthritis (10). According to
the World Health Organization, the prevalence
of osteoarthritis in the Iranian urban population
is reported to be about 19.3% (5). The findings
of a similar study in Iran show that osteoarthritis
is higher in the Iranian population than in
the other studied populations, and the prevalence
in women is more than in men (11). OA costs
60 billion dollars a year for the US economy
(12). This disease is one of the main causes
of functional impairment and has greatly influenced
people’s lives, including their mobility,
independence, and daily activities, resulting
in limited recreational activities, sports,
and work (13). The results of a 2004 study in
Iran investigating 200 patients with osteoarthritis
showed that high BMI, high age, and live in
a village were the main factors affecting the
inability of these patients (14). Sex also plays
a major role in this issue, about 2.3% to 3.4%
of the knee OA patients are female (15).
The inflammation process also plays an important
role in osteoarthritis, and cytokines such as
IL-1 beta, IL-6, tumor necrosis factor, and
IL-15 play a role in this disease (16-17). The
disease is divided into two primary and secondary
forms. In the primary type, the degeneration
process and joint destruction occurs without
previous anomalies. Its main cause is unknown,
usually it is seen in individuals over 40 years
of age with slow progressive and multiple arthroplasty,
and is seen through normal or abnormal pressure
on the weak joint (8, 15). Secondary osteoarthritis
is followed by an underlying cause such as fractures,
bone and joint injuries, infections, rheumatoid
arthritis, and congenital and metabolic diseases
(18).
In terms of pathology, this disease is caused
by three biological, mechanical and biomechanical
causes. Symptoms begin with mild pain in one
or more joints and gradually intensify. This
pain is improved with exertion and relaxation,
with the advancement of pain, it develops and
joint stiffness lasts for a few minutes (19).
Failure to use a joint with OA due to pain results
in rapid atrophy of the muscles around the joint,
and therefore, lead to muscle loss, which is
one of the most important factors for joint
support. Eventually, in the last stages of the
disease or when there is severe pain (20), it
disturbs patients’ quality of life, and
ultimately leads to surgery such as joint replacement
(21). Pain is a multidimensional phenomenon
that has physical, psychological, social, and
spiritual components, and is, in fact, a kind
of unpleasant sensory and psychological experience
that is associated with actual or potential
tissue damage and it is expressed with a series
of words from people who experience it (22).
The lack of management of chronic pain affects
the physical and mental condition of individuals,
decreases their quality of life and that of
their families, and on the other hand, along
with the physical and psychological disabilities,
it imposes a significant cost to the economic
resources of countries, health systems and insurance
(23). In addition to the direct medical costs
caused by pain, it imposes the following indirect
costs, such as complications of therapeutic
measures, the number of days someone cannot
handle, movement restrictions, being useless
and ineffective, functional disorders, pain-related
disabilities, and compensation for these disabilities
on the individual and the community (24).
In industrialized countries and developing
countries attention to knee osteoarthritis is
an important cause of pain and disability, the
loss of proper joint performance, and joint
instability and deformity are increasing (25).
Therefore, several therapeutic approaches have
been proposed for the treatment or improvement
of this disease. Multiple treatments for this
disease include medication, lifestyle changes,
weight loss, muscle strengthening, using cane,
brace, heel wedge and surgical procedures. All
of these methods have a sedative effect and
only delay the onset of the disease (26). The
standard of care and treatment is multifactorial
in osteoarthritis, and often involves physical
therapy, prescribing and taking anti-inflammatory
drugs, intracranial injection of hyaluronic
acid (visco-supplementation) and arthroscopic
surgery. New studies also show no therapeutic
effect left alone (27).
Unfortunately, no definitive treatment for this
disease has been found despite the many used
therapeutic methods. Therefore, given the long
duration, high financial costs, widespread side
effects, non-steroidal anti-inflammatory drugs,
and finally, the symptoms of the disease lead
to limitation of movement and severe disability
and loss of muscle performance and muscle weakness;
therapeutic goals of the disease should include
reducing pain and weakness, improving performance
and range of motion, and facilitating day-to-day
activities. Treatment of the disease includes
medical treatments and non-pharmacological treatments
including physiotherapy. Another promising treatment
that has recently been used to treat musculoskeletal
pain is prolotherapy (28, 29). Prolotherapy
is a selective therapeutic and complementary
injection for chronic musculoskeletal pain.
Prolotherapy techniques and injected intra-articular
materials are very different and are related
to the patient’s condition, severity of
symptoms and clinical manifestations of patients.
Prolotherapy involves infusion of a very small
amount of an anti-inflammatory or sclerosis
agent into the tendon, inflamed or painful joint
or ligament (30).
It is assumed that prolotherapy leads to stimulate
recovery in chronic soft tissue injuries; typically,
dextrose hypertonic is used in prolotherapy
for intramuscular injection (30). The study
of Reeves et al. (2003) showed that the pain
of the patients was significantly decreased
after the injection of into the hip (31). Jo
et al. also found that intra-joint 15% dextrose
injection can reduce knee pain in these individuals
(32). A study by Rabago et al showed that in
adults with osteoarthritis, using intra-articular
dextrose reduces pain, rigidity and increased
function of patients without side effects (33).
Knee osteoarthritis can result in severe physical
and mental disability, and the therapeutic goals
in this disease include reducing weakness, improving
performance, reducing pain, increasing the range
of motion, reducing the morning stiffness of
the joints, and facilitating the daily functioning
of life (34) and due to the need to find safe,
simple and inexpensive non-surgical treatments
to reduce pain and improve the function of patients
with knee osteoarthritis and the limited number
of studies in this field, this study aimed to
investigate the effect of dextrose injection
on the control of pain associated with knee
osteoarthritis in patients referred to Taleghani
Hospital (2017).
The study was a single-blind clinical trial.
The research population was all patients with
knee osteoarthritis, who were selected by available
sampling method from 80 knee osteoarthritis
patients referred to Taleghani Hospital. They
were randomly divided into two groups: 15% dextrose
injection and injection of hypertonic dextrose
25% divided. The sample size was 80 individuals
based on similar research (p0.05) and a test
power of 80%. The criteria for entering the
study included: unilateral idiopathic OA of
the knee, age range of 45-75 years, walking
ability, local knee pain with a score of more
than 5 based on VAS criteria and exit criteria
including: other knee diseases, hip joint OA,
and ankle sprain, radicular pain due to lumbar
spine disorders, intraocular effusion, history
of physiotherapy and intra-articular injection
in the past 6 months, psycho-mental diseases,
knee necrotic tissue, infection and tissue in
the blood, neurological, sensory and motor disorders,
history of knee surgery and obesity. Ethical
Criteria of this study was approved by the ethics
committee of Shahid Beheshti University of Medical
Sciences.
Method of implementation
After diagnosis of the patient as an appropriate
case, education about the method of implementation
and the benefits and possible complications
of participating in the project, written consent
was taken from the patient. They were informed
about the necessity of regular referral for
follow up, but that it was not imposed. The
intervention was performed without the cost
to the patient. Before the intervention, a questionnaire
was filled out including patient’s demographic
information, such as: gender, age, occupation,
involved side (upper leg), history of previous
treatments, and history of underlying illness
and the duration of symptoms. In addition to
providing an educational brochure on how to
inject, the time for referrals to perform tests
and the next visit was presented face to face.
Regarding moral considerations, the patient
was assured that they could be excluded from
the study whenever they wished, and that their
failure to cooperate with the doctor and the
hospital would not affect their treatment and
all patient information would be kept confidential.
The injection procedure was performed in such
a way that the patient was placed in a supine
position and marked with a knee flexion of 10-15
degrees on the medial side of the knee, marking
the injection area, and then the injection site
was disinfected with Povidone iodine and the
injected area was anesthetized with 1 ml 1%
Lidocaine solution and using needle number 25-27
after aspiration and ensuring proper placement
of needle for intra-articular injection (35).
In the 25% dextrose group, solution was made
of 5 cc 50% dextrose and 5 cc 1% lidocaine.
Then, 6 cc of this 25% dextrose solution was
injected into the patient’s joint and injection
was performed with the inferomedial approach
(33). In the 15% dextrose group, solution was
made of 6.75 cc 50% dextrose and 4.5 cc of 1%
lidocaine and 11.25 cc of normal saline 0.9%.
Then, 0.5 cc of this solution was 15% dextrose
that was injected as subdermal with peppering
technique with needle number 25 in the bone
ligament. There were 15 injections for each
patient (33). This injection was performed at
the beginning of the study, the first week,
the fifth week and the ninth week. The completion
of the WOMAC questionnaire and the implementation
of the VAS scale were performed before the intervention,
and in the first week, the fifth week, the ninth
week and the thirteenth week. To measure the
variables, the Western Ontario and McMaster
Universities (WOMAC) index and the VAS Scale
(Visual Analogue Scale) were used as follows.
Visual Analogue Scale
The visual analogue scale (VAS) indicates the
pain of the patients in general. This scale
is plotted as a 10 cm line, and the degree of
pain is graded from zero to 10 cm. The zero
number does not show any pain, 1 to 3 mild pain,
4 to 6 moderate pain and 7 to 10 severe pain
[36]. The internal reliability of this tool
has been reported as 0.85 to 0.95 (37).
Functional questionnaire of WOMAC
The WOMAC functional questionnaire consists
of 24 questions, 5 questions regarding pain,
2 questions related to stiffness and 16 questions
regarding the performance of patients with osteoarthritis.
The score for each question varies from zero
to four. This criterion is scored from zero
to 96. If the patient has no problem, then,
the score is zero and if they have a maximum
problem, score will be 96. Validity and reliability
of this tool have been investigated by Ebrahimzadeh
et al. and has been validated in the Persian
language. Cronbach’s alpha was estimated
0.9 in Persian language (5).
In analyzing data, the mean, standard deviations,
frequencies, tables and charts were used to
categorize and summarize the collected data.
In the study of statistical pre-requisites,
the number of observations per distribution
was used to test the natural distribution of
the data using the Kolmogorov-Smirnov test.
Regarding the existence of statistical hypotheses,
independent t-test and two way-analysis of variance
with repeated measures (p0.05) and using the
Statistical package of version 22 were used.
The
participants
in
the
present
study
consisted
of
48
(60%)
women
and
32
(40%)
men.
The
age
range
of
patients
was
(45-75)
years
and
the
mean
age
was
64.3
years.
VAS
variable
The
results
of
Kolmogorov-Smirnov
test
showed
that
the
distribution
of
data
was
normal
(P>
0.05).
T-test
showed
that
there
was
no
significant
difference
in
VAS
scale
between
the
two
groups
before
intervention
(t=0.781,
p>
0.05).
Two-way
analysis
of
variance
(week
×
group)
of
3×2
was
used
to
analyze
the
data.
The
results
are
presented
in
Table
1.
Table
1:
The
results
of
variance
analysis
of
VAS
scale
in
two
groups
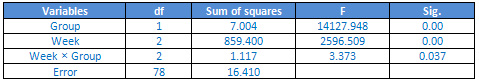
The
findings
showed
that
the
main
effect
of
the
group
(F2.78
=
14127.948,
p<0/05),
the
main
effect
of
week
(F2.78
=
2596.509,
p<0.05)
and
the
interaction
between
the
group
and
the
week
was
significant.
The
significance
effect
of
the
group
means
that
there
is
a
significant
difference
between
the
two
groups
in
the
visual
analogue
scale.
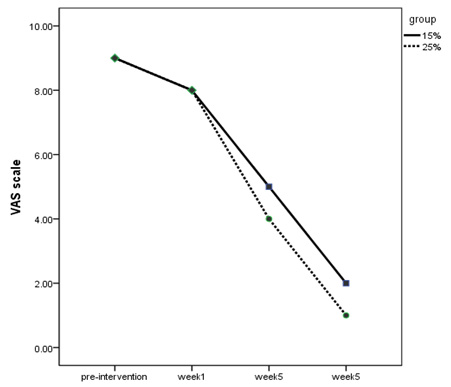
According
to
Chart
1,
the
group
of
25%
Dextrose
injection
experienced
more
pain
relief
than
the
15%
group.
Significance
of
the
weeks
of
treatment
meant
that
during
the
weeks
of
injection,
the
process
of
pain
reduction
continued
significantly
(Figure
1).
Figure
1:
VAS
scale
of
the
two
groups
in
the
weeks
of
treatment
WOMAC
variable
The
results
of
Kolmogorov-Smirnov
test
showed
that
the
distribution
of
data
was
normal
(P>0.05).
T-test
showed
that
there
was
no
significant
difference
in
the
WOMAC
scale
between
the
two
groups
before
the
intervention
(t
=
0.841,
p>0.05).
Two-way
analysis
of
variance
(week
×
group)
of
3×2
was
used
to
analyze
the
data.
The
results
are
presented
in
Table
2.
Table
2:
The
results
of
variance
analysis
of
WOMAC
scale
in
two
groups
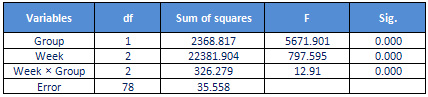
The
findings
showed
that
the
main
effect
of
the
group
(F2.78
=
5671/901,
p
<0.05),
the
main
effect
of
week
(F2.78
=
797/595,
p
<0.05)
and
the
interaction
between
the
group
and
the
week
was
significant.
The
significance
of
the
effect
of
the
group
means
that
there
is
a
significant
difference
between
the
two
groups
on
the
WOMAC
scale.
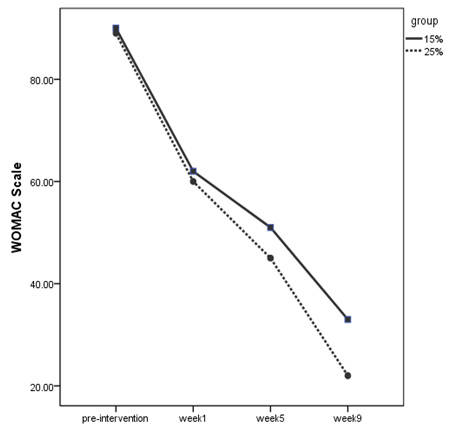
According
to
Figure
2,
it
can
be
said
that
25%
dextrose
injection
group
had
a
better
experience.
Significantly,
the
weeks
of
treatment
means
that
during
the
weeks
of
injection,
the
improvement
in
performance
was
significantly
increased
(Figure.
2).
Figure
2:
WOMAC
scale
of
the
two
groups
in
the
weeks
of
treatment
The
purpose
of
this
study
was
to
investigate
the
effect
of
dextrose
injection
on
pain
control
associated
with
knee
osteoarthritis.
The
findings
showed
that
injection
of
15%
and
25%
of
dextrose
had
a
significant
effect
on
the
visual
scale
of
pain
and
function
of
patients
so
that
during
treatment,
scales
showed
improvement
in
treatment
in
these
patients.
Also,
other
findings
showed
that
injection
of
25%
dextrose
compared
to
15%
had
a
significant
effect
on
visual
scale
of
pain
and
function
of
patients.
These
findings
are
consistent
with
the
results
of
Reeves
and
Hassanin
(2004),
Rabago
(2012),
Jo
(2004),
Reeves
and
Hassanin
(2000),
Hashemi
(2015)
and
Reeves
(2003).
For
example,
the
findings
of
Rabago
(2012)
showed
that
in
adults
with
osteoarthritis,
using
intra-arterial
dextrose
reduces
pain,
stiffness
and
increased
function
of
the
patients
without
any
side
effects
(33).
Joe
et
al.
(2004)
showed
that
the
pain
of
patients
was
significantly
reduced
by
15%
dextrose
injection.
They
also
concluded
that
intra-articular
injection
of
15%
dextrose
can
reduce
knee
pain
in
these
individuals
(32).
In
another
study,
Hashemi
et
al.
(2015)
attempted
to
compare
the
effect
of
ozone
therapy
and
dextrose
injection
in
patients
with
osteoarthritis.
They
evaluated
the
patients
using
the
WOMAC
and
VAS
scales.
The
findings
showed
that
in
both
groups,
pain
significantly
decreased
and
function
was
significantly
increased.
They
concluded
that
both
treatments
were
effective
in
reducing
pain
and
increasing
the
function
of
patients
(38).
In
subsequent
studies,
Reeves
and
Hassanein
(2000)
evaluated
the
effect
of
10%
dextrose
on
osteoarthritis
of
fingers.
After
six
months
of
follow
up,
they
found
that
in
the
dextrose
group,
a
significant
improvement
was
observed
in
the
case
of
xylocaine
group
during
fingers
movement
and
joint
flexion,
but
there
was
no
significant
improvement
in
pain
during
rest
and
recovery.
Another
study
on
knee
osteoarthritis
and
anterior
AC
ligation
showed
significant
improvement
in
pain
and
knee
swelling
and
flexion,
but
in
the
ACL
group,
there
was
no
significant
improvement
in
instability
(40).
Also,
Hassanein
and
Reeves
(2002)
conducted
a
study
on
patients
with
joint
instability
associated
with
ACL
rupture.
Their
findings
showed
that
in
patients
with
a
three
year
follow
up,
there
was
a
significant
decrease
in
pain
during
walking,
joint
swelling
and
joint
flexion
(40).
In
another
study
for
the
treatment
of
osteoarthritis,
finger
joints
used
10%
dextrose
over
two
months,
which
was
associated
with
beneficial
therapeutic
effects
(41).
In
another
study,
it
has
been
reported
that
in
third
world
countries
where
knee
insertion
surgery
is
not
available,
in
contrast
to
symptomatic
patients,
exercise,
physiotherapy
or
NSAIDs
are
prescribed.
The
researchers
found
that
10%
dextrose
could
modify
ACL
ligament
laxity,
which
was
not
associated
with
rupture,
and
also
prevented
gradual
salivation
after
surgery
in
joints
with
a
potential
displacement
(42).
The
mechanism
of
dextrose
effect
is
that
injection
of
a
stimulant
such
as
dextrose
into
a
damaged
joint,
possibly
with
local
inflammatory
reactions,
may
lead
to
an
increase
in
blood
flow
around
the
joint
and
damaged
tissue,
thereby
causing
self-repair
in
that
area.
The
dextrose
effect
has
another
mechanism
of
effect
(43).
They
showed
that
in
treatment
with
10%
Dextrose,
the
response
rate,
the
accumulation
and
tightening
of
the
uterus,
was
significantly
better
than
oxytocin
treatment
(40
units
per
liter).
These
researchers
argued
that
the
mechanism
of
dextrose
effect
is
that
since
the
activity
of
the
sympathetic
nervous
system
and
the
level
of
adrenalin
of
the
blood
increases
at
an
advanced
age,
this
increase
in
adrenalin
increases
the
level
of
cAMP
by
binding
to
beta
receptors
and
thus,
activates
the
protein
kina
dependent
to
cAMP,
which
in
turn
has
a
moderating
role
in
kinase
adhesion
to
the
myosin-like
chain
and
calcium-calmodulin
molecule,
and
therefore,
result
in
reduction
in
the
contractile
power
of
the
smooth
muscle.
Hence,
at
an
advanced
age,
it
is
necessary
to
increase
the
level
of
dextrose
and
consequently
increase
the
level
of
ATP
for
exposure
to
high
levels
of
catecholamines
to
help
accumulate
and
tighten
the
uterus.
According
to
the
results,
it
can
be
concluded
that
the
mechanism
of
the
effect
of
Dextrose
Prolotherapy
is
direct
effects,
osmotic
and
inflammatory
growth.
Dextrose
injection
with
a
concentration
of
less
than
10%
directly
promotes
cell
and
tissue
proliferation
without
inflammatory
reaction
and
a
high
concentration
of
10%
results
in
an
extracellular
osmotic
gradient
at
the
injection
site
resulting
in
loss
of
intracellular
and
lyse
cellular
cells
and
invasion
of
growth
factors
and
inflammatory
cells
that
start
the
wound
healing
cascade
in
that
particular
area.
Dextrose
is
an
ideal
proliferrant
because
it
is
water-soluble
and
is
a
mixture
of
blood
that
can
be
safely
injected
into
several
areas
and
in
large
quantities,
and
the
final
result
is
the
insertion
of
new
collagen
into
damaged
tissues
such
as
Ligaments
and
tendons.
When
extracellular
dextrose
concentrations
reach
5%,
normal
cells
begin
to
proliferate
and
produce
a
number
of
growth
factors
such
as
platelet
growth
factor,
TGF-,
epidermal
growth
factor,
basal
growth
factor
fibroblast
growth
factor,
insulin-like
growth
factor,
and
connective
tissue
growth
factor
that
repairs
the
tendon,
ligaments
and
other
soft
tissues.
Finally,
according
to
human
and
animal
studies,
dextrose
Prolotherapy
has
a
significant
effect
on
musculoskeletal
pain,
disability
and
cost
of
treatment.
Major
complications
from
dextrose
have
not
been
reported,
and
include
mostly
side
effects
of
injection
(pain
in
injection
site,
hematoma,
infection,
and
skin
pigmentation)
(38,
39).
According
to
the
findings
of
this
study,
the
use
of
Dextrose
Prolotherapy
is
a
simple,
safe,
inexpensive,
available
and
uncomplicated
method
for
other
remedies
in
these
patients,
which
has
been
confirmed
by
other
studies.
1.
Jordan
JM,
et
al.
The
impact
of
arthritis
in
rural
populations.
Arthritis
&
Rheumatism.
1995;
8(4):
242-250.
2.
Gordon,
A.,
P.,
the
Five
Pillars
of
Pain
Management:
A
Systematic
Approach
to
Treating
Osteoarthritis.
Osteoarthritis
and
Cartilage,
2006.
14:
p.
S193.
3.
Kurtai,
Y.,
et
al.,
Reliability,
construct
validity
and
measurement
potential
of
the
ICF
comprehensive
core
set
for
osteoarthritis.
BMC
musculoskeletal
disorders,
2011.
12(1):
p.
255.
4.
Buckwalter,
J.A.
and
N.E.
Lane,
Athletics
and
osteoarthritis.
The
American
Journal
of
Sports
Medicine,
1997.
25(6):
p.
873-881.
5.
Ebrahimzadeh,
M.H.,
et
al.,
The
Western
Ontario
and
McMaster
Universities
Osteoarthritis
Index
(WOMAC)
in
Persian
Speaking
Patients
with
Knee
Osteoarthritis.
Archives
of
bone
and
joint
surgery,
2014.
2(1):
p.
57.
6.
Neogi,
T.,
et
al.,
Association
between
radiographic
features
of
knee
osteoarthritis
and
pain:
results
from
two
cohort
studies.
Bmj,
2009.
339.
7.
Felson,
D.T.,
et
al.,
Osteoarthritis:
new
insights.
Part
1:
the
disease
and
its
risk
factors.
Annals
of
internal
medicine,
2000.
133(8):
p.
635-646.
8.
Sowers,
M.,
Epidemiology
of
risk
factors
for
osteoarthritis:
systemic
factors.
Current
opinion
in
rheumatology,
2001.
13(5):
p.
447-451.
9.
Williams,
F.M.
and
T.D.
Spector,
Biomarkers
in
osteoarthritis.
Arthritis
Research
and
Therapy,
2008.
10(1):
p.
10.
10.
Issa,
S.N.
and
L.
Sharma,
Epidemiology
of
osteoarthritis:
an
update.
Current
rheumatology
reports,
2006.
8(1):
p.
7-15.
11.
Haq,
S.A.
and
F.
Davatchi,
Osteoarthritis
of
the
knees
in
the
COPCORD
world.
International
journal
of
rheumatic
diseases,
2011.
14(2):
p.
122-129.
12.
Buckwalter,
J.A.,
C.
Saltzman,
and
T.
Brown,
The
impact
of
osteoarthritis:
implications
for
research.
Clinical
orthopaedics
and
related
research,
2004.
427:
p.
S6-S15.
13.
Salavati,
M.,
et
al.,
Validation
of
a
Persian-version
of
Knee
injury
and
Osteoarthritis
Outcome
Score
(KOOS)
in
Iranians
with
knee
injuries.
Osteoarthritis
and
Cartilage,
2008.
16(10):
p.
1178-1182.
14.
Shakibi,
M.R.,
Relationship
Between
Pain
and
Disability
in
Osteoarthritis
Patients:
Is
Pain
a
Predictor
for
Disability?
J.
Med.
Sci,
2004.
4(2):
p.
115-119.
15.
Moskowitz,
R.W.,
Osteoarthritis:
diagnosis
and
medical/surgical
management.
2001:
WB
Saunders
Company.
16.
Waldmann,
T.A.,
Targeting
the
interleukin-15
system
in
rheumatoid
arthritis.
Arthritis
&
Rheumatism,
2005.
52(9):
p.
2585-2588.
17.
Nishimoto,
N.,
et
al.,
Treatment
of
rheumatoid
arthritis
with
humanized
anti–interleukin-6
receptor
antibody:
A
multicenter,
double-blind,
placebo-controlled
trial.
Arthritis
&
Rheumatism,
2004.
50(6):
p.
1761-1769.
18.
Cole,
B.J.
and
C.D.
Harner,
Degenerative
arthritis
of
the
knee
in
active
patients:
evaluation
and
management.
Journal
of
the
American
Academy
of
Orthopaedic
Surgeons,
1999.
7(6):
p.
389-402.
19.
Moskowitz,
R.W.,
Osteoarthritis:
diagnosis
and
medical/surgical
management.
2007:
Lippincott
Williams
&
Wilkins.
20.
Mease,
P.J.,
et
al.,
Pain
mechanisms
in
osteoarthritis:
understanding
the
role
of
central
pain
and
current
approaches
to
its
treatment.
The
Journal
of
rheumatology,
2011.
38(8):
p.
1546-1551.
21.
Bennell,
K.,
et
al.,
Efficacy
of
physiotherapy
management
of
knee
joint
osteoarthritis:
a
randomised,
double
blind,
placebo
controlled
trial.
Annals
of
the
Rheumatic
Diseases,
2005.
64(6):
p.
906-912.
22.
Pizzo,
P.A.
and
N.M.
Clark,
Alleviating
suffering
101–pain
relief
in
the
United
States.
N
Engl
J
Med,
2012.
366(3):
p.
197-199.
23.
Gran,
S.V.,
L.S.
Festvåg,
and
B.T.
Landmark,
‘Alone
with
my
pain–it
can’t
be
explained,
it
has
to
be
experienced’.
A
Norwegian
in-depth
interview
study
of
pain
in
nursing
home
residents.
International
journal
of
older
people
nursing,
2010.
5(1):
p.
25-33.
24.
Brennan,
F.,
D.B.
Carr,
and
M.
Cousins,
Pain
management:
a
fundamental
human
right.
Anesthesia
&
Analgesia,
2007.
105(1):
p.
205-221.
25.
Foley,
A.,
et
al.,
Does
hydrotherapy
improve
strength
and
physical
function
in
patients
with
osteoarthritis—a
randomised
controlled
trial
comparing
a
gym
based
and
a
hydrotherapy
based
strengthening
programme.
Annals
of
the
Rheumatic
Diseases,
2003.
62(12):
p.
1162-1167.
26.
Andreoli,
T.E.,
et
al.,
Andreoli
and
Carpenter’s
Cecil
essentials
of
medicine.
2010:
Elsevier
Health
Sciences.
27.
Samson,
D.J.,
et
al.,
Treatment
of
primary
and
secondary
osteoarthritis
of
the
knee.
Evid
Rep
Technol
Assess
(Full
Rep),
2007.
157(157):
p.
1-157.
28.
Hassan,
F.,
et
al.,
The
effectiveness
of
prolotherapy
in
treating
knee
osteoarthritis
in
adults:
a
systematic
review.
British
Medical
Bulletin,
2017.
122(1):
p.
91-108.
29.
Reeves,
K.D.
and
K.
Hassanein,
Randomized
prospective
double-blind
placebo-controlled
study
of
dextrose
prolotherapy
for
knee
osteoarthritis
with
or
without
ACL
laxity.
Alternative
therapies
in
health
and
medicine,
2000.
6(2):
p.
68.
30.
Rabago,
D.,
A.
Slattengren,
and
A.
Zgierska,
Prolotherapy
in
primary
care
practice.
Primary
Care:
Clinics
in
Office
Practice,
2010.
37(1):
p.
65-80.
31.
Reeves,
K.D.
and
K.M.
Hassanein,
Long
term
effects
of
dextrose
prolotherapy
for
anterior
cruciate
ligament
laxity.
Alternative
therapies
in
health
and
medicine,
2003.
9(3):
p.
58.
32.
Jo,
D.
and
M.
Kim,
The
effects
of
Prolotherapy
on
knee
joint
pain
due
to
ligament
laxity.
Journal
of
the
Korean
Pain
Society,
2004.
17(1):
p.
47-50.
33.
Rabago,
D.,
et
al.,
Hypertonic
dextrose
injections
(prolotherapy)
for
knee
osteoarthritis:
results
of
a
single-arm
uncontrolled
study
with
1-year
follow-up.
The
Journal
of
Alternative
and
Complementary
Medicine,
2012.
18(4):
p.
408-414.
34.
Ross,
S.M.,
Osteoarthritis
of
the
knee:
An
integrative
therapies
approach.
Holistic
nursing
practice,
2011.
25(6):
p.
327-331.
35.
Hashemi,
S.M.,
et
al.,
Intra-articular
hyaluronic
acid
injections
vs.
dextrose
prolotherapy
in
the
treatment
of
osteoarthritic
knee
pain.
Tehran
University
of
Medical
Sciences,
2012.
70(2).
36.
Jensen,
M.P.,
P.
Karoly,
and
S.
Braver,
The
measurement
of
clinical
pain
intensity:
a
comparison
of
six
methods.
Pain,
1986.
27(1):
p.
117-126.
37.
Mokkink,
L.B.,
et
al.,
Construct
validity
of
the
DynaPort®
KneeTest:
a
comparison
with
observations
of
physical
therapists.
Osteoarthritis
and
Cartilage,
2005.
13(8):
p.
738-743.
38.
Hashemi,
M.,
et
al.,
The
effects
of
prolotherapy
with
hypertonic
dextrose
versus
prolozone
(intraarticular
ozone)
in
patients
with
knee
osteoarthritis.
Anesthesiology
and
pain
medicine,
2015.
5(5).
39.
Hashemi,
SM.,
et
al.,
Intra-articular
hyaluronic
acid
injections
vs.
dextrose
prolotherapy
in
the
treatment
of
osteoarthritic
knee
pain.
Tehran
Univ
Med
J,
2012.
70(2).119-125
40.
Kim
SR,
Stitik
TP,
Foye
PM:
critical
review
of
prolotherapy
growth
factor-beta
and
basic
fibroblast
growth
factor.
J
Bone
for
osteoarthritis
A
physiatric
perspective
2004;
83:
379-
Joint
Sur.
1995;
77:
543-5439.
41.
Reeves
KD,
Hassanein
K.
Randomized
prospective
double-blind
placebo-controlled
study
of
dextrose
prolotherapy
for
knee
osteoarthritis
with
or
without
ACL
laxity.
Altern
Ther
Health
Med.
2000;
6(2):68-74,
77-80
42.
Reeves
KD,
Harris
AL.
Recurrent
dislocation
of
total
knee
prostheses
in
a
large
patient:
Case
report
of
dextrose
proliferant
use.
Arch
Phys
Med
Rehabil
1997;
78:1039.
43.
Rafiee,
M.R.,
Samimi,
M.,
Nouraldini,
M.,
&
Mousavi,
S.GH.A.
Comparison
of
the
effect
of
10%
dextrose
and
oxytocin
40
units
per
liter
on
uterine
contraction
after
cesarean
section.
Yafte,
2006,
8(1),
13-18
|

