|
The effect of eye drop
excipients against Acanthamoeba polyphaga
by AlamarBlueTM assay
Jeehan Alestada
(1)
Roua Abulkassimb (2)
Ruwida Omarc (3)
(1) Department of Micobiology, Faculty of Medicine,
Kuwait University, Jabriya, Kuwait;
(2) Strathclyde Institute of Pharmacy and Biomedical
Sciences, University of Strathclyde, 161 Cathedral
Street, Glasgow, G4 0RE, UK.
(3) Strathclyde Institute of Pharmacy and Biomedical
Sciences, University of Strathclyde, 27 Taylor
street, Glasgow, G4 0NR, UK.
Correspondence:
Jeehan Alestad, PhD.
Department of Microbiology, Parasitology
Faculty of Medicine, Kuwait University
Telephone 00965 51500100
Email: j.alestad@hsc.edu.kw
|
Abstract
Objective: Based
on the reduction of alamarBlueTM, we have
therefore screened a variety of such eye
drop excipients used for bacterial keratitis
in order to identify any candidates that
show inhibitory activity against Acanthamoeba
polyphaga, one of the protozoal species
responsible for the Acanthamoeba
Keratitis.
Subjects and Methods: Acanthamoeba
keratitis is a serious eye infection which
is notoriously difficult to treat successfully.
The currently employed drugs have significant
disadvantages in that they have to be
administered at hourly intervals for extended
periods of time. The AlamarBlueTM assay
has been optimized for determination of
selected eye drop excipients efficacy
against potentially pathogenic strain,
Acanthamoeba polyphaga.
Results: The
most effective agents were found to be
fusidic acid and framycetin sulfate, with
a combination of the two providing a reduction
in A. polyphaga metabolic activity
of around 75%.
Conclusion: These
eye drop excipients can serve as new sources
for the discovery and development of much
needed new antimicrobials for both
Acanthamoeba keratitis and bacterial
keratitis.
Key words:
AlamarBlue; Acanthamoeba keratitis;
Acanthamoeba polyphaga
|
Acanthamoeba keratitis is a serious
eye infection caused by Acanthamoeba
species of protozoa. These protozoa are present
in the majority of water bodies, including sea
water, sewage, soil and tap water. Previously
a relatively rare condition, prevalence of Acanthamoeba
keratitis is increasing [1]. This is mainly
caused by the growing use of contact lenses,
with approximately 85% of infections occurring
in contact lens users [2, 4]. Acanthamoeba
polyphaga is one of the two main protozoa
species responsible for the condition. This
microorganism has two stages to its life cycle,
a rapidly reproducing trophozoite phase followed
by encystation to form a robust double-layered
cyst that allows survival under harsh conditions
such as the presence of toxic chemicals [5].
This cyst stage provides a significant hurdle
in the treatment of Acanthamoeba keratitis,
with most drugs having demonstrated limited
activity against it [6].
Chlorhexidine and polyhexamethylene biguanide
(PHMB) are currently the standard treatments
for the condition, being active against both
the trophozoite and cyst phases of the organism.
Diamidines such as hexamidine are sometimes
used in conjunction with these; however, their
use alone should be avoided owing to the development
of resistance [4, 7]. A further issue associated
with these agents is the necessity to apply
them at hourly intervals for extended periods
of time. Novel and more effective drugs for
treating Acanthamoeba keratitis are therefore
highly sought after. Phosphocholines have shown
some promise, with inhibitory activity against
Acanthamoeba and other parasites being demonstrated
in vitro and in animal tests [8-11). A number
of potential targets for new treatments have
been identified. These include various components
of the cell membrane, mitochondria, and protein
synthesis pathways [12]. Such targets present
a wide variety of agents that could be screened
for activity against A. polyphaga. A
selection of possible drugs of interest is already
used in eye drop form; however, their efficacies
for specifically treating Acanthamoeba
keratitis have not yet been investigated.
In the present study, we have screened eight
components of commercially available eye drop
solutions in order to identify any agents with
the potential for treating the condition. To
allow for rapid analysis of all excipients simultaneously,
we employed an alamarBlueTM microplate assay
that has been previously verified for use in
analysing the response of A. polyphaga
to inhibitory drugs [13].
Culture of A. polyphaga
A. polyphaga (strain 1501/18) was obtained from
Culture Collection of Algae and Protozoa (Lincoln,
London). The cells were cultured in medium supplemented
with 20% mycological peptone, 0.9% maltose,
and 1% penicillin, streptomycin, and amphotericin
B (all Sigma-Aldrich, Pennsylvania, USA). They
were incubated in 75 cm2
flasks at room temperature, when cultures reached
90-95% confluence.
Determination of optimal
seeding densities for Acanthamoeba
After harvesting, A. polyphaga were diluted
in culture medium to give a stock solution containing
8.0 × 105 cells/ml.
Different concentrations of cells were used
for tests of varying length. For the 24 hour
test, 100 ul aliquots
of a solution of 8.0 × 104
cells/ml were added to the wells of a 96-well
plate. The seeding densities of A. polyphaga
that attained close to 100% alamarBlue reduction
were determined in assays conducted for total
periods of 24 and 96 hours.
AlamarBlue growth
inhibition assay
A stock solution of A. polyphaga was
prepared at 8.0 × 105
cells/ml in culture medium. Inhibition tests
were carried out for two different time periods.
For the 24 hour test, 100 ul
aliquots of a solution of 8.0 × 104
cells/ml were added to the wells of a 96-well
plate. For the 96 hour test, 100 u
l of a solution
of 1.25 × 103 cells/ml
were added to each well. Each test was carried
out in triplicate, with the experiment carried
out twice. The different eye drop excipients
to be tested were added to the wells at the
concentrations related to the compound solubility.
Synergistic effects of the eye drop excipients
were determined by using different combinations
of the agents. The cultures were incubated at
room temperature for the duration of the test
period, after which 10 ul
of alamarBlue reagent (Life Technologies,
Renfrew, UK) was added to each well and the
plates were incubated for a further 6 hours.
The absorbance of the solutions was then measured
at 570 nm and 600 nm using Gemini EM Microplate
Reader (Molecular Devices, Sunnyvale, USA).
The percentage reduction of alamarBlue was then
calculated according to the following equation:
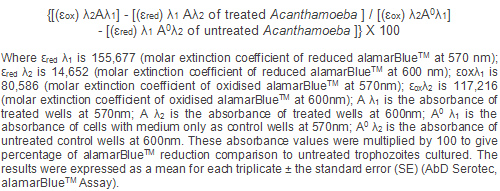
Statistics
All tests were carried out twice in triplicate.
As the results of the two experiments were highly
similar, statistical analysis was carried out
on the data from one experiment. Values are
expressed as the mean with the standard error
(SE). Statistical significance was calculated
using the Mann-Whitney U test, with a p-value
of <0.05 considered to be significant. Statistical
analysis was performed using the GRAPH PAD PRISM
5 software.
The
addition
of
chloramphenicol
to
the
A.
polyphaga
cultures
resulted
in
a
significant
dose-dependent
decrease
in
alamarBlue
reduction
by
the
cells,
but
only
for
the
96
hour
test
(Figure
1A).
No
inhibitory
activity
was
found
for
the
24
hour
culture.
On
the
other
hand,
fusidic
acid
had
a
large
inhibitory
effect
for
both
culture
lengths,
with
the
concentrations
at
which
50%
inhibition
was
achieved
(IC50
values)
being
0.125%
and
0.062%
for
the
24
hour
and
96
hour
experiments,
respectively
(Figure
1B).
Framycetin
sulfate
also
displayed
inhibitory
activity,
although
this
was
only
significant
enough
to
calculate
an
IC50
when
the
culture
was
carried
out
for
96
hours
(IC50:
5.0%;
(Figure
1C).
Gramicidin
caused
a
level
of
inhibition
for
both
culture
durations,
with
an
IC50
of
5.0%
calculated
for
the
96
hour
experiments
(Figure
1D).
Ciprofloxacin
showed
some
activity
at
the
highest
concentrations,
but
this
reached
no
more
than
a
percentage
reduction
of
alamarBlue
of
85%
(Figure
1E).
Neither
benzalkonium
chloride
nor
sodium
carboxymethyl
cellulose
displayed
any
inhibitory
activity
against
A.
polyphaga
at
any
concentration
for
either
culture
length
(Figure
1
F
and
H).
Phenylmercuric
nitrate
on
the
other
hand,
displayed
a
level
of
activity
at
the
higher
concentrations,
with
the
effect
being
more
pronounced
for
the
96
hour
culture
(Figure
1G).
The
effect
was
not
significant
enough
for
an
IC50
to
be
calculated,
however.
When
different
combinations
of
the
eye
drop
excipients
were
tested
for
activity
against
A.
polyphaga,
the
combination
of
fusidic
acid
and
benzalkonium
chloride
(Figure
2A)
appeared
to
provide
much
the
same
response
to
that
of
fusidic
acid
alone
(Figure
1B).
Again,
high
inhibitory
activity
was
found
for
both
culture
lengths,
with
perhaps
a
slight
increase
in
activity
when
the
highest
concentration
of
benzalkonium
chloride
was
present
for
the
96
hour
experiment.
The
combination
of
chloramphenicol
and
phenylmercuric
nitrate
produced
no
inhibition
of
the
microorganism
for
the
24
hour
culture
(Figure
2B).
For
the
96
hour
culture,
however,
some
activity
was
evident
at
the
higher
chloramphenicol
concentrations.
The
shape
of
the
curves
closely
mirrored
that
of
when
phenylmercuric
nitrate
was
tested
alone
(Figure
1G),
but
the
concentration
of
this
excipient
had
no
effect
on
the
level
of
inhibition.
Another
combination
of
excipients
that
was
tested
was
gramicidin
with
framycetin
sulfate.
When
tested
alone,
both
of
these
agents
demonstrated
inhibitory
activity
against
A.
polyphaga
in
both
the
24
hour
and
96
hour
cultures
(Figure
1C
and
D).
Together,
a
small
additive
effect
can
be
seen,
with
the
level
of
inhibition
being
greatest
when
the
highest
concentrations
of
each
excipient
were
used
in
combination
(Figure
2C).
While
chloramphenicol
alone
only
showed
inhibitory
activity
for
the
96
hour
culture,
in
combination
with
framycetin
sulfate,
activity
was
evident
for
both
lengths
of
experiment
(Figure
2D).
For
the
shorter
of
the
two
cultures,
the
inhibitory
activity
was
higher
for
the
combination
of
excipients
than
that
when
framycetin
was
tested
alone.
The
maximum
level
of
alamarBlue
reduction
for
the
highest
concentration
of
framycetin
sulphate
(5
mg/ml)
was
approximately
55%,
while
this
decreased
to
around
40%
when
combined
with
5%
chloramphenicol.
For
the
96
hour
culture,
alamarBlue
reduction
reached
a
low
of
approximately
25%
for
the
combination
of
the
highest
concentrations
of
the
two
excipients.
This
was
much
lower
than
the
70%
and
45%
found
for
chloramphenicol
and
framycetin
sulfate
alone,
respectively
(Figure
1A
and
C).
There
is
an
increasing
need
to
develop
novel
agents
against
A.
polyphaga,
one
of
the
protozoal
species
responsible
for
Acanthamoeba
keratitis.
The
task
of
identifying
such
compounds
is
ongoing;
however,
to
date,
no
study
has
evaluated
the
efficacy
of
agents
already
used
in
commercial
eye
drops.
Having
already
been
demonstrated
to
be
safe
for
ophthalmologic
use,
such
compounds
could
rapidly
gain
regulatory
approval
for
the
treatment
of
this
potentially
blinding
condition.
Chloramphenicol
displays
broad
bacteriostatic
activity
against
both
gram
positive
and
gram
negative
bacteria
by
inhibiting
protein
synthesis
via
irreversible
binding
to
the
50S
subunit
of
the
ribosome.
It
has
long
been
used
to
treat
bacterial
conjunctivitis
[14],
and
has
recently
demonstrated
anti-yeast
properties
[15].
We
found
that
alamarBlue
reduction
by
A.
polyphaga
was
inhibited
by
the
compound,
but
only
when
the
cells
were
cultured
in
its
presence
for
96
hours.
This
indicates
that
chloramphenicol
works
slowly
against
the
organism,
requiring
a
certain
length
of
time
in
order
to
achieve
an
effect.
Fusidic
acid
is
another
bacteriostatic
compound,
displaying
activity
against
gram
positive
bacteria.
It
works
by
inhibiting
protein
synthesis
via
prevention
of
turnover
of
elongation
factor
G
from
the
ribosome
[16],
and
is
used
in
the
treatment
of
bacterial
conjunctivitis
[17].
We
found
that
the
agent
produced
significant
inhibitory
activity
against
A.
polyphaga
in
both
the
24
hour
and
96
hour
cultures.
This
demonstrates
that
despite
its
narrow
scope
as
an
antibacterial,
it
is
a
promising
candidate
for
the
treatment
of
Acanthamoeba
keratitis.
Framycetin
sulfate
is
a
broad
spectrum
aminoglycoside
antibacterial
that
works
by
inhibiting
protein
synthesis
via
ribosomal
binding.
It
is
active
against
gram
negative
and
some
gram
positive
bacteria,
but
has
not
been
demonstrated
to
have
any
antifungal
activity.
Whilst
there
are
no
reports
on
the
effect
of
this
agent
on
any
protozoal
species,
another
aminoglycoside
antibacterial,
paromomycin,
has
demonstrated
activity
against
the
Leishmania
species
of
protozoa
[18-20].
We
found
that
framycetin
sulfate
inhibited
alamarBlue
reduction
by
A.
polyphaga,
with
a
more
significant
effect
evident
for
the
longer
96
hour
culture.
The
data
therefore
suggest
that
this
compound
is
another
potential
therapy
for
Acanthamoeba
keratitis,
and
warrants
further
study.
Gramicidin
is
an
antibacterial
that
causes
cell
death
by
increasing
the
permeability
of
the
cell
membrane
leading
to
leakage
of
small
molecules
such
as
monovalent
ions
and
amino
acids.
To
date,
reports
of
the
activity
of
gramicidin
have
been
limited
to
gram
positive
bacteria,
with
no
evidence
of
antifungal
or
antiprotozoal
activity
when
used
alone.
Here,
we
found
that
the
compound
had
a
level
of
activity
against
A.
polyphaga,
with
this
being
greater
for
the
96
hour
culture
in
comparison
with
the
24
hour.
Ciprofloxacin
is
a
fluoroquinolone
broad
spectrum
antibacterial
that
is
used
to
treat
conjunctivitis,
keratitis,
and
corneal
ulcers
[21].
It
works
by
hindering
cell
division
via
inhibition
of
DNA
topoisomerases
[22].
The
compound
has
also
displayed
activity
against
Leishmania
or
topoisomerases
present
in
this
organism
[23,
24].
Here,
we
found
that
ciprofloxacin
demonstrated
a
low
level
of
activity
against
A.
polyphaga,
with
similar
activity
profiles
for
the
two
different
culture
lengths.
Such
limited
activity
indicates
that
this
compound
would
not
be
useful
for
the
treatment
of
Acanthamoeba
keratitis.
Benzalkonium
chloride
is
a
quaternary
ammonium
salt
preservative
used
in
many
forms
of
eye
drops
and
artificial
tears.
It
works
as
an
antibacterial
by
binding
to
the
negatively
charged
cell
membrane
and
increasing
its
permeability,
resulting
in
leakage
of
monovalent
ions
and
subsequently,
cell
death.
No
inhibitory
activity
against
A.
polyphaga
was
found
in
the
present
study,
for
either
culture
length.
This
is
in
contrast
to
the
data
published
by
Tu
et
al.,
who
demonstrated
significant
in
vitro
activity
of
benzalkonium
chloride
against
three
species
of
Acanthamoeba,
among
them
A.
polyphaga
[25).
Activity
was
high
after
just
an
hour,
even
at
concentrations
much
lower
than
those
used
in
the
present
work.
Zanetti
et
al.
reported
activity
of
the
compound
against
A.
castellanii,
another
organism
responsible
for
Acanthamoeba
keratitis,
both
in
trophozoite
form
and
cyst
form
[26].
These
conflicting
results
suggest
that
further
tests
should
be
carried
out
before
benzalkonium
chloride
is
discounted
as
a
treatment
for
the
condition.
Phenylmercuric
nitrate
is
another
preservative
used
in
eye
drops.
It
displays
both
antibacterial
and
antifungal
activity
as
a
result
of
increasing
the
permeability
of
the
cell
membrane
[27,
29].
We
found
that
the
compound
was
active
against
A.
polyphaga
at
the
higher
concentrations,
with
a
greater
effect
for
the
96
hour
culture.
This
indicates
that
phenylmercuric
nitrate
may
be
useful
in
the
treatment
of
Acanthamoeba
keratitis
if
the
dosing
can
be
sustained
over
a
number
of
days.
The
final
eye
drop
excipient
that
we
tested
was
the
viscosity
modifier,
sodium
carboxymethyl
cellulose.
As
expected,
this
compound
demonstrated
no
inhibitory
activity
against
A.
polyphaga.
Fusidic
acid
eye
drops
often
contain
benzalkonium
chloride
as
a
preservative;
we
therefore
tested
these
two
agents
in
combination
in
order
to
determine
if
there
was
an
additive
effect.
The
activity
of
the
combination
was
generally
the
same
as
that
of
fusidic
acid
alone.
The
only
exception
to
this
was
a
slightly
greater
activity
when
the
highest
concentration
of
benzalkonium
chloride
was
used
in
the
96
hour
culture.
Importantly,
no
detrimental
effect
was
found,
indicating
that
commercially
available
fusidic
acid
eye
drops
may
be
a
potential
treatment
option
for
Acanthamoeba
keratitis.
Another
commonly
found
combination
of
agents
in
eye
drops
is
chloramphenicol
and
phenylmercuric
nitrate.
For
the
24
hour
culture,
this
combination
appeared
to
have
no
inhibitory
activity
against
A.
polyphaga,
despite
phenylmercuric
nitrate
alone
showing
some
activity
in
the
earlier
experiments.
This
is
likely
due
to
the
low
concentrations
of
this
compound
that
were
added
to
the
chloramphenicol
for
this
particular
test.
The
activity
of
the
phenylmercuric
nitrate
was
only
found
at
the
higher
concentrations
that
were
tested
in
the
single
compound
experiment.
It
is
also
possible
that
the
presence
of
chloramphenicol
lowered
the
activity
of
the
phenylmercuric
nitrate.
The
data
taken
from
the
96
hour
culture
also
point
to
this
possibility
as
the
level
of
A.
polyphaga
inhibition
was
almost
the
same
as
that
seen
for
the
chloramphenicol
alone,
with
no
additional
effect
found
on
the
addition
of
the
phenylmercuric
nitrate.
This
combination
of
excipients
does
not
appear
to
be
a
potentially
effective
treatment
for
Acanthamoeba
keratitis.
Gramicidin
and
framycetin
sulfate
are
often
used
in
combination
for
treating
eye
infections.
We
found
that
the
inhibitory
activity
of
this
combination
was
greater
than
that
found
for
either
agent
alone.
For
the
96
h
culture
in
particular,
alamarBlue
reduction
decreased
to
approximately
25%,
one
of
the
lowest
values
found
in
the
present
study.
The
data
therefore
suggest
that
the
commercially
available
eye
drops
that
utilise
this
combination
could
be
effectively
used
for
the
treatment
of
Acanthamoeba-based
infections.
We
also
investigated
the
combination
of
framycetin
sulfate
and
chloramphenicol.
For
the
24
hour
culture,
while
chloramphenicol
alone
had
not
demonstrated
any
activity
in
the
prior
tests,
on
the
addition
of
even
a
small
amount
of
framycetin
sulfate,
there
was
an
increase
in
A.
polyphaga
inhibition.
This
additive
effect
was
even
more
evident
for
the
96
hour
culture,
with
alamarBlue
reduction
decreasing
to
around
25%
when
the
highest
concentrations
of
the
two
compounds
were
used
together.
This
combination
of
agents
appears
to
be
a
promising
treatment
option
for
Acanthamoeba
keratitis
and
warrants
further
evaluation.
We
have
demonstrated
significant
activity
against
A.
polyphaga
of
a
number
of
compounds
that
are
currently
used
as
active
ingredients
of
preservatives
in
commercially
available
eye
drops.
These
preliminary
data
indicate
that
further
investigation
into
some
of
these
agents
may
lead
to
additional
treatment
options
for
Acanthamoeba
keratitis.
As
these
excipients
have
already
been
approved
for
ocular
use,
there
is
potential
for
new
combinations
of
them
to
be
rapidly
introduced
to
the
market.
Conflict
Of
Interest
The
author
of
this
publication
receives
research
support
from
Public
Authority
for
Agriculture
Affairs
and
Fish
Resources
-
Al-rabia,
Kuwait
City..
The
terms
of
this
arrangement
have
been
reviewed
and
approved
by
the
University
of
Kuwait
in
accordance
with
its
policy
on
objectivity
in
research.
1.
Lorenzo-Morales
J,
Khan
NA,
Walochnik
J.
An
update
on
Acanthamoeba
keratitis:
diagnosis,
pathogenesis
and
treatment.
Parasite
(Paris,
France)
2015;22:10.
2.
Lorenzo-Morales
J,
Martin-Navarro
CM,
Lopez-Arencibia
A,
Arnalich-Montiel
F,
Pinero
JE,
Valladares
B.
Acanthamoeba
keratitis:
an
emerging
disease
gathering
importance
worldwide?
Trends
in
parasitology
2013;29:181-187.
3.
Pacella
E,
La
Torre
G,
De
Giusti
M,
Brillante
C,
Lombardi
AM,
Smaldone
G,
Lenzi
T,
Pacella
F.
Results
of
case-control
studies
support
the
association
between
contact
lens
use
and
Acanthamoeba
keratitis.
Clinical
ophthalmology
(Auckland,
NZ)
2013;7:991-994.
4.
Dart
JK,
Saw
VP,
Kilvington
S.
Acanthamoeba
keratitis:
diagnosis
and
treatment
update
2009.
American
journal
of
ophthalmology
2009;148:487-499
e482.
5.
Alkharashi
M,
Lindsley
K,
Law
HA,
Sikder
S.
Medical
interventions
for
Acanthamoeba
keratitis.
The
Cochrane
database
of
systematic
reviews
2015;2:CD010792.
6.
Schuster
FL,
Visvesvara
GS.
Opportunistic
amoebae:
challenges
in
prophylaxis
and
treatment.
Drug
resistance
updates
:
reviews
and
commentaries
in
antimicrobial
and
anticancer
chemotherapy
2004;7:41-51.
7.
Hay
J,
Kirkness
CM,
Seal
DV,
Wright
P.
Drug
resistance
and
Acanthamoeba
keratitis:
the
quest
for
alternative
antiprotozoal
chemotherapy.
Eye
(London,
England)
1994;8
(
Pt
5):555-563.
8.
Croft
SL,
Seifert
K,
Duchene
M.
Antiprotozoal
activities
of
phospholipid
analogues.
Molecular
and
biochemical
parasitology
2003;126:165-172.
9.
Polat
ZA,
Obwaller
A,
Vural
A,
Walochnik
J.
Efficacy
of
miltefosine
for
topical
treatment
of
Acanthamoeba
keratitis
in
Syrian
hamsters.
Parasitology
research
2012;110:515-520.
10.
Polat
ZA,
Walochnik
J,
Obwaller
A,
Vural
A,
Dursun
A,
Arici
MK.
Miltefosine
and
polyhexamethylene
biguanide:
a
new
drug
combination
for
the
treatment
of
Acanthamoeba
keratitis.
Clinical
&
experimental
ophthalmology
2014;42:151-158.
11.
Seifert
K,
Duchene
M,
Wernsdorfer
WH,
Kollaritsch
H,
Scheiner
O,
Wiedermann
G,
Hottkowitz
T,
Eibl
H.
Effects
of
miltefosine
and
other
alkylphosphocholines
on
human
intestinal
parasite
Entamoeba
histolytica.
Antimicrobial
agents
and
chemotherapy
2001;45:1505-1510.
12.
Roberts
CW,
Henriquez
FL.
Drug
target
identification,
validation,
characterisation
and
exploitation
for
treatment
of
Acanthamoeba
(species)
infections.
Experimental
parasitology
2010;126:91-96.
13.
McBride
J,
Ingram
PR,
Henriquez
FL,
Roberts
CW.
Development
of
colorimetric
microtiter
plate
assay
for
assessment
of
antimicrobials
against
Acanthamoeba.
Journal
of
clinical
microbiology
2005;43:629-634.
14.
McGhee
CN,
Anastas
CN.
Widespread
ocular
use
of
topical
chloramphenicol:
is
there
justifiable
concern
regarding
idiosyncratic
aplastic
anaemia?
The
British
journal
of
ophthalmology
1996;80:182-184.
15.
Joseph
MR,
Al-Hakami
AM,
Assiry
MM,
Jamil
AS,
Assiry
AM,
Shaker
MA,
Hamid
ME.
In
vitro
anti-yeast
activity
of
chloramphenicol:
A
preliminary
report.
Journal
de
mycologie
medicale
2015;25:17-22.
16.
Borg
A,
Holm
M,
Shiroyama
I,
Hauryliuk
V,
Pavlov
M,
Sanyal
S,
Ehrenberg
M.
Fusidic
acid
targets
elongation
factor
G
in
several
stages
of
translocation
on
the
bacterial
ribosome.
The
Journal
of
biological
chemistry
2015;290:3440-3454.
17.
Golledge
C.
Fusidic
acid
in
other
infections.
International
journal
of
antimicrobial
agents
1999;12
Suppl
2:S11-15.
18.
Scott
JA,
Davidson
RN,
Moody
AH,
Grant
HR,
Felmingham
D,
Scott
GM,
Olliaro
P,
Bryceson
AD.
Aminosidine
(paromomycin)
in
the
treatment
of
leishmaniasis
imported
into
the
United
Kingdom.
Transactions
of
the
Royal
Society
of
Tropical
Medicine
and
Hygiene
1992;86:617-619.
19.
Thakur
CP,
Kanyok
TP,
Pandey
AK,
Sinha
GP,
Zaniewski
AE,
Houlihan
HH,
Olliaro
P.
A
prospective
randomized,
comparative,
open-label
trial
of
the
safety
and
efficacy
of
paromomycin
(aminosidine)
plus
sodium
stibogluconate
versus
sodium
stibogluconate
alone
for
the
treatment
of
visceral
leishmaniasis.
Transactions
of
the
Royal
Society
of
Tropical
Medicine
and
Hygiene
2000;94:429-431.
20.
Maarouf
M,
Lawrence
F,
Croft
SL,
Robert-Gero
M.
Ribosomes
of
Leishmania
are
a
target
for
the
aminoglycosides.
Parasitology
research
1995;81:421-425.
21.
Chisari
G,
Reibaldi
M.
Ciprofloxacin
as
treatment
for
conjunctivitis.
Journal
of
chemotherapy
(Florence,
Italy)
2004;16:156-159.
22.
Smith
A,
Pennefather
PM,
Kaye
SB,
Hart
CA.
Fluoroquinolones:
place
in
ocular
therapy.
Drugs
2001;61:747-761.
23.
Cortazar
TM,
Coombs
GH,
Walker
J.
Leishmania
panamensis:
comparative
inhibition
of
nuclear
DNA
topoisomerase
II
enzymes
from
promastigotes
and
human
macrophages
reveals
anti-parasite
selectivity
of
fluoroquinolones,
flavonoids
and
pentamidine.
Experimental
parasitology
2007;116:475-482.
24.
Romero
IC,
Saravia
NG,
Walker
J.
Selective
action
of
fluoroquinolones
against
intracellular
amastigotes
of
Leishmania
(Viannia)
panamensis
in
vitro.
The
Journal
of
parasitology
2005;91:1474-1479.
25.
Tu
EY,
Shoff
ME,
Gao
W,
Joslin
CE.
Effect
of
low
concentrations
of
benzalkonium
chloride
on
Acanthamoebal
survival
and
its
potential
impact
on
empirical
therapy
of
infectious
keratitis.
JAMA
ophthalmology
2013;131:595-600.
26.
Zanetti
S,
Fiori
PL,
Pinna
A,
Usai
S,
Carta
F,
Fadda
G.
Susceptibility
of
Acanthamoeba
castellanii
to
contact
lens
disinfecting
solutions.
Antimicrobial
agents
and
chemotherapy
1995;39:1596-1598.
27.
Debbasch
C,
Brignole
F,
Pisella
PJ,
Warnet
JM,
Rat
P,
Baudouin
C.
Quaternary
ammoniums
and
other
preservatives'
contribution
in
oxidative
stress
and
apoptosis
on
Chang
conjunctival
cells.
Investigative
ophthalmology
&
visual
science
2001;42:642-652.
28.
Xu
Y,
He
Y,
Li
X,
Gao
C,
Zhou
L,
Sun
S,
Pang
G.
Antifungal
effect
of
ophthalmic
preservatives
phenylmercuric
nitrate
and
benzalkonium
chloride
on
ocular
pathogenic
filamentous
fungi.
Diagnostic
microbiology
and
infectious
disease
2013;75:64-67.
29.
Kaur
IP,
Lal
S,
Rana
C,
Kakkar
S,
Singh
H.
Ocular
preservatives:
associated
risks
and
newer
options.
Cutaneous
and
ocular
toxicology
2009;28:93-103.
|

