|
Left renal atrophy in
sickle cell diseases
Mehmet Rami
Helvaci (1)
Ramazan Davran (2)
Mursel Davarci (3)
Orhan Ekrem Muftuoglu (4)
Lesley Pocock (5)
(1) Medical Faculty of the Mustafa Kemal University,
Antakya, Professor of Internal Medicine, M.D.
(2) Medical Faculty of the Mustafa Kemal University,
Antakya, Assistant Professor of Radiology, M.D.
(3) Medical Faculty of the Mustafa Kemal University,
Antakya, Associated Professor of Urology, M.D.
(4) Medical Faculty of the Dicle University,
Diyarbakir, Professor of Internal Medicine,
M.D.
(5) Lesley Pocock, medi+WORLD International,
Australia
Correspondence:
Mehmet Rami Helvaci, M.D.
Medical Faculty of the Mustafa Kemal University,
31100, Serinyol, Antakya, Hatay, Turkey
Phone: 00-90-326-2291000 (Internal 3399) Fax:
00-90-326-2455654
Email: mramihelvaci@hotmail.com
|
Abstract
Background: We
tried to understand whether or not there
is a difference in occurrence of renal
atrophy between the left and right sides
in sickle cell diseases (SCDs).
Methods: All
patients with SCDs were enrolled into
the study.
Results: The
study included 311 patients (153 females).
There were seven cases (2.2%) with left
renal atrophy against one case (0.3%)
with right renal atrophy (p<0.001).
Associated thalassemias were detected
in 44.0% and splenomegaly in 12.5% of
the patients. There was digital clubbing
in 6.4%, chronic obstructive pulmonary
disease in 4.8%, leg ulcers in 12.8%,
stroke in 7.0%, chronic renal disease
in 8.6%, pulmonary hypertension in 11.8%,
cirrhosis in 3.5%, coronary heart disease
in 8.0%, and exitus in 5.7% of the patients.
Conclusion:
Renal atrophy is significantly higher
on the left side in SCDs. Splenomegaly
induced flow disorders in left renal vessels,
structural anomalies of the left renal
vein including nutcracker syndrome and
passage behind the aorta, and possibly
the higher arterial pressure of left kidney
due to the shorter distance to heart as
an underlying cause of endothelial damage
induced atherosclerosis, may be some of
the possible causes. Because of the higher
prevalences of left varicocele probably
due to drainage of left testicular vein
into the left renal vein, high prevalences
of associated thalassemias with SCDs as
a cause of splenomegaly, and tissue ischemia
and infarctions induced edematous splenomegaly
in early lives of the SCDs cases, splenomegaly
induced flow disorders of left renal vein
may be the most significant cause among
them.
Key words:
Sickle cell diseases, splenomegaly, left
renal vein, left renal atrophy
|
Arterio- or atherosclerosis, but not venosclerosis,
is an inflammatory process, probably developing
secondary to the much higher arterial pressure
induced chronic endothelial damage all over
the body. It may be the main cause of aging
induced end-organ failures in human beings (1,2).
It is a systemic and irreversible process initiating
at birth, and accelerated by many factors. The
accelerating factors known for the moment are
collected under the heading of metabolic syndrome.
Some reversible components of the syndrome are
overweight, hypertriglyceridemia, hyperbetalipoproteinemia,
dyslipidemia, white coat hypertension, impaired
fasting glucose, impaired glucose tolerance,
and smoking for the development of terminal
consequences such as obesity, diabetes mellitus
(DM), hypertension (HT), coronary heart disease
(CHD), chronic obstructive pulmonary disease
(COPD), cirrhosis, chronic renal disease (CRD),
peripheric artery disease (PAD), stroke, and
other end-organ failures (3-8). Sickle cell
diseases (SCDs) are a prototype of the accelerated
atherosclerosis (9,10), by which we can observe
terminal consequences of the metabolic syndrome
very early in life. SCDs are caused by homozygous
inheritance of the hemoglobin S (Hb S). Hb S
causes erythrocytes to change their normal elastic
structures to hard bodies. Actually, rigidity
instead of shapes of the erythrocytes is the
central pathology of the SCDs. The rigidity
process is probably present in whole life, but
exaggerated with stresses. The erythrocytes
can take their normal elastic structures after
normalization of the stresses, but after repeated
attacks of rigidity, they become hard bodies,
permanently. The rigid cells induced chronic
endothelial damage causes tissue ischemia, infarctions,
and end-organ failures even in the absence of
obvious vascular occlusions due to the damaged
and edematous endothelium. We tried to understand
whether or not there is a difference according
to the renal atrophy between the left and right
sides in the SCDs patients.
The study was performed in the Hematology
Service of the Mustafa Kemal University between
March 2007 and May 2013. All patients with SCDs
were enrolled into the study. SCDs are diagnosed
by the hemoglobin electrophoresis performed
via high performance liquid chromatography (HPLC)
method. Their medical histories including smoking
habit, regular alcohol consumption, leg ulcers,
and stroke were learnt. Cases with a history
of three pack-year were accepted as smokers
and cases with a history of regular alcohol
consumption with one drink a day for three years
were accepted as alcoholics. A check up procedure
including serum iron, total iron binding capacity,
serum ferritin, serum creatinine value on three
occasions, hepatic function tests, markers of
hepatitis viruses A, B, and C and human immunodeficiency
virus, an electrocardiogram, a Doppler echocardiogram,
an abdominal ultrasonography, and a computed
tomography of the brain were performed. Cases
with acute painful crisis or any other inflammatory
event were treated at first, and then the spirometric
pulmonary function tests to diagnose COPD, the
Doppler echocardiography to measure the systolic
pressure of pulmonary artery, renal and hepatic
function tests, and measurement of serum ferritin
level were performed on the silent phase. Renal
atrophies were detected ultrasonographically.
The criterion for diagnosis of COPD is post-bronchodilator
forced expiratory volume in 1 second/forced
vital capacity of less than 70% (11). Systolic
pressure of the pulmonary artery of 40 mmHg
or higher during the silent phase is accepted
as pulmonary hypertension (12). CRD is diagnosed
with a permanently elevated serum creatinine
level of 1.3 mg/dL or higher on the silent phase.
Cases with renal transplantation were put into
the CRD group. Cirrhosis is diagnosed with hepatic
function tests, ultrasonographic findings, ascites,
and histologic procedure in case of requirement.
Digital clubbing is diagnosed with the ratio
of distal phalangeal diameter to interphalangeal
diameter of higher than 1.0 and with the presence
of Schamroth's sign (13,14). Associated thalassemias
are diagnosed by serum iron, total iron binding
capacity, serum ferritin, and the hemoglobin
electrophoresis performed via HPLC method. A
stress electrocardiography was performed in
cases with an abnormal electrocardiogram and/or
angina pectoris. A coronary angiography was
obtained just for the stress electrocardiography
positive cases. So CHD was diagnosed either
with the Doppler echocardiographic findings
as the movement disorders of the cardiac walls
or angiographically. Mann-Whitney U test, Independent-Samples
t test, and comparison of proportions were used
as the methods of statistical analyses.
The
study
included
311
patients
with
the
SCDs
(153
females
and
158
males).
The
mean
ages
of
them
were
28.2
±
9.2
(8-59)
versus
29.9
±
9.6
(6-58)
years
in
females
and
males,
respectively
(p>0.05).
Interestingly,
there
were
seven
cases
(2.2%)
of
the
left
renal
atrophy
against
only
one
case
(0.3%)
of
the
right
renal
atrophy
(p<0.001)
among
the
study
cases
(Table
1).
On
the
other
hand,
associated
thalassemias
were
detected
in
44.0%,
splenomegaly
in
12.5%,
and
autosplenectomy
in
48.5%
of
the
SCDs
patients.
Although
smoking
was
observed
in
7.0%
of
the
patients,
there
was
only
one
case
(0.3%)
with
regular
alcohol
consumption.
Additionally,
there
were
digital
clubbing
in
6.4%,
COPD
in
4.8%,
leg
ulcers
in
12.8%,
stroke
in
7.0%,
CRD
in
8.6%,
pulmonary
hypertension
in
11.8%,
cirrhosis
in
3.5%,
CHD
in
8.0%,
and
exitus
in
5.7%
of
the
cases
with
the
SCDs.
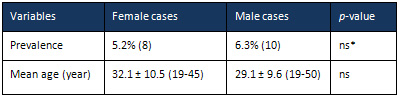
Prevalence
of
mortality
were
similar
in
both
genders
(5.2%
versus
6.3%
in
females
and
males,
respectively,
p>0.05),
and
mean
ages
of
the
mortal
cases
were
32.1
versus
29.1
years
in
females
and
males,
respectively
(p>0.05)
(Table
2).
On
the
other
hand,
five
of
the
CRD
cases
were
on
hemodialysis,
and
one
with
right
renal
transplantation.
Histologic
procedure
for
the
diagnosis
of
cirrhosis
was
not
required
in
any
case.
Although
antiHCV
was
positive
in
two
of
the
cirrhotics,
HCV
RNA
was
detected
as
negative
by
polymerase
chain
reaction
in
both.
The
solitary
case
of
regular
alcohol
consumption
was
not
cirrhotic
at
the
time
of
study.
Table
1:
Sickle
cell
patients
with
associated
disorders
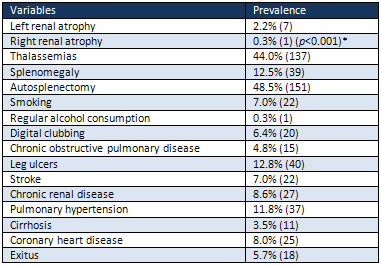
Table
2:
Features
of
the
mortal
cases
Nephrons
are
the
basic
functional
units
of
the
kidneys
located
in
the
renal
parenchyma,
and
each
kidney
contains
about
one
million
nephrons.
Renal
atrophy
is
characterized
by
shrinkage
of
kidneys
due
to
loss
of
nephrons.
Loss
of
nephrons
also
causes
shrinkages
of
the
renal
arteries
and
veins,
secondarily.
Renal
diseases,
urinary
tract
obstructions,
or
acute
or
chronic
pyelonephritis
may
cause
renal
atrophy.
Reflux
nephropathy
is
characterized
by
renal
damage
due
to
the
backflow
of
urine,
and
it
may
also
cause
renal
atrophy.
Renal
atrophy
may
also
be
caused
by
the
obstruction
of
urinary
tract
due
to
an
increased
pressure
on
it,
or
compression
of
the
intrarenal
veins
or
arteries.
Obstructive
uropathy
causes
a
higher
urinary
pressure
within
the
kidneys
causing
damage
to
the
nephrons.
Although
the
various
etiologies,
probably
renal
ischemia
is
the
most
frequent
cause
of
the
renal
atrophy.
Probably
the
most
common
cause
of
renal
ischemia
is
the
systemic
atherosclerosis,
and
CRD
due
to
the
systemic
atherosclerosis
is
common
in
elderlies.
Although
the
younger
mean
ages,
we
detected
CRD
in
8.6%
of
all
cases
in
the
present
study,
since
the
SCDs
are
an
accelerated
systemic
atherosclerotic
process.
SCDs
are
accelerated
systemic
atherosclerotic
processes
(9)
initiating
at
birth,
and
by
which
we
can
observe
final
consequences
of
the
systemic
atherosclerosis
which
began
30
or
40
years
earlier
in
life.
Actually
name
of
the
syndrome
should
be
'Rigid
Cell
Induced
Chronic
Endothelial
Dysfunction'
instead
of
the
SCDs
or
sickle
cell
anemia
since
we
cannot
observe
the
sickle
cells
in
the
peripheric
blood
samples
of
cases
with
additional
thalassemias,
easily.
On
the
other
hand,
the
rigidity
of
the
erythrocytes
is
the
main
problem
instead
of
their
shapes
or
severity
of
anemia.
The
rigid
cells
induced
chronic
endothelial
damage
causes
tissue
ischemia,
infarction,
and
end-organ
failures
even
in
the
absence
of
obvious
vascular
occlusions
on
the
chronic
background
of
damaged
and
edematous
endothelium
all
over
the
body.
Even
there
were
patients
with
severe
vision
or
hearing
loss
among
the
present
study
cases.
The
digital
clubbing
and
recurrent
leg
ulcers
may
also
indicate
the
chronic
tissue
hypoxia
in
such
patients.
Due
to
the
reversibility
of
digital
clubbing
and
leg
ulcers
with
the
hydroxyurea
treatment,
the
chronic
endothelial
damage
is
probably
prominent
at
the
microvascular
level
as
in
diabetic
microangiopathies,
and
reversible
to
some
extent.
Although
large
arteries
and
arterioles
are
especially
important
for
blood
carriage,
capillaries
are
more
important
for
tissue
oxygenation.
So
passage
of
the
rigid
cells
through
the
endothelial
cells
cause
damage
on
the
capillaries.
Reversibility
of
the
process
may
probably
be
more
in
early
years
of
life
but
it
gets
an
irreversible
nature
over
time.
Thus
endothelial
cells
all
over
the
body
are
edematous
and
swollen
due
to
the
destructive
process
as
in
splenomegaly
seen
in
early
years
of
life.
But
the
ischemic
process
terminates
with
tissue
fibrosis
and
shrinkage
all
over
the
body
as
in
autosplenectomy.
Even
there
were
four
cases
with
total
teeth
loss
and
one
case
with
right
ovarian
atrophy
among
the
study
cases.
The
solitary
case
of
right
renal
atrophy
may
also
be
explained
by
the
mechanism.
On
the
other
hand,
anemia
probably
is
not
the
cause
of
the
end-organ
failures
in
the
SCDs,
since
we
cannot
observe
any
shortened
survival
in
the
thalassemia
minor
cases
although
the
presence
of
a
moderate
anemia.
Although
the
mean
survivals
were
42
and
48
years
for
males
and
females
for
the
SCDs
in
the
literature
(15),
they
were
29.1
and
32.1
years
in
males
and
females
in
the
present
study,
respectively.
The
great
differences
between
the
survival
may
be
secondary
to
the
initiation
of
hydroxyurea
in
infancy
in
such
countries
(16).
The
accelerated
atherosclerotic
process
can
also
affect
the
renal
arteries,
and
may
lead
to
poor
perfusion
of
the
kidneys
leading
to
reduced
renal
function
and
failure.
The
right
renal
artery
is
longer
than
the
left
because
of
the
location
of
the
aorta,
since
the
aorta
is
found
on
the
left
side
of
the
body.
Additionally,
the
right
renal
artery
is
lower
than
the
left
because
of
the
lower
position
of
the
right
kidney.
So
the
left
kidney
possibly
has
a
relatively
higher
arterial
pressure
due
to
the
shorter
distance
to
heart
as
an
underlying
cause
of
endothelial
damage
induced
atherosclerosis.
But
according
to
our
opinion,
the
accelerated
atherosclerotic
process
alone
cannot
explain
the
significantly
higher
prevalence
of
renal
atrophy
on
the
left
side
(2.2%
versus
0.3%,
p<0.001)
in
the
present
study.
The
left
renal
atrophy
has
also
been
reported
in
the
literature
(17).
On
the
other
hand,
the
very
high
prevalences
of
associated
thalassemias
(44.0%)
and
splenomegaly
(12.5%)
with
the
SCDs
cases
may
be
important
for
the
explanation,
since
spleen
and
left
kidney
are
closely
related
organs
which
may
also
be
observed
with
the
development
of
varicose
veins
from
the
left
renal
vein
at
the
splenic
hilus
in
cirrhotic
cases.
Any
pressure
on
the
left
kidney
as
in
splenomegaly
cases
may
cause
torsion
of
the
renal
vein,
and
prevents
its
drainage.
We
especially
think
about
the
drainage
problems
at
the
venous
level
due
to
the
much
higher
arterial
pressure
that
cannot
be
obstructed
easily
and
the
much
higher
prevalence
of
varicocele
in
the
left
side
in
males
(18-20).
Varicocele
is
a
dilatation
of
pampiniform
venous
plexus
within
the
scrotum.
It
occurs
in
15-20%
of
all
males
and
40%
of
infertile
males,
since
researchers
documented
a
recurrent
pattern
of
low
sperm
count,
poor
motility,
and
predominance
of
abnormal
sperm
forms
in
varicocele
cases
(21,22).
Varicoceles
are
much
more
common
(nearly
80%
to
90%)
in
the
left
side
due
to
several
anatomic
factors
including
angle
at
which
the
left
testicular
vein
enters
the
left
renal
vein,
lack
of
effective
antireflux
valves
at
the
juncture
of
left
testicular
vein
and
left
renal
vein,
the
nutcracker
syndrome,
and
some
other
left
renal
vein
anomalies
such
as
passage
behind
the
aorta.
The
nutcracker
syndrome
results
mostly
from
the
compression
of
the
left
renal
vein
between
the
abdominal
aorta
and
superior
mesenteric
artery,
although
other
variants
exist
(23).
It
may
cause
hematuria
and
left
flank
pain
(24).
Since
the
left
gonad
drains
via
the
left
renal
vein,
it
can
also
result
in
left
testicular
pain
in
men
or
left
lower
quadrant
pain
in
women
(25).
Nausea
and
vomiting
may
result
due
to
compression
of
the
splanchnic
veins
(25).
An
unusual
manifestation
of
the
nutcracker
syndrome
includes
varicocele
formation
and
varicose
veins
in
the
lower
limbs
(26).
Another
study
has
shown
that
the
nutcracker
syndrome
is
a
frequent
finding
in
varicocele
patients
(27),
so
it
should
be
routinely
searched
in
cases
with
left
varicocele.
As
a
conclusion,
the
renal
atrophy
is
significantly
higher
on
the
left
side
in
the
SCDs
cases.
Splenomegaly
induced
flow
disorders
in
the
left
renal
vessels,
structural
anomalies
of
the
left
renal
vein
including
nutcracker
syndrome
and
passage
behind
the
aorta,
and
possibly
the
higher
arterial
pressure
of
the
left
kidney
due
to
the
shorter
distance
to
heart
as
an
underlying
cause
of
endothelial
damage
induced
atherosclerosis
may
be
some
of
the
possible
causes.
Because
of
the
higher
prevalences
of
left
varicocele
probably
due
to
drainage
of
left
testicular
vein
into
the
left
renal
vein,
high
prevalences
of
associated
thalassemias
with
the
SCDs
as
a
cause
of
splenomegaly,
and
tissue
ischemia
and
infarctions
induced
edematous
splenomegaly
in
early
lives
of
the
SCDs
cases,
splenomegaly
induced
flow
disorders
of
the
left
renal
vein
may
be
the
most
significant
cause
among
them.
1.
Helvaci
MR,
Aydin
LY,
Aydin
Y.
Digital
clubbing
may
be
an
indicator
of
systemic
atherosclerosis
even
at
microvascular
level.
HealthMED
2012;
6:
3977-3981.
2.
Helvaci
MR,
Aydin
Y,
Gundogdu
M.
Smoking
induced
atherosclerosis
in
cancers.
HealthMED
2012;
6:
3744-3749.
3.
Eckel
RH,
Grundy
SM,
Zimmet
PZ.
The
metabolic
syndrome.
Lancet
2005;
365:
1415-1428.
4.
Helvaci
MR,
Kaya
H,
Gundogdu
M.
Association
of
increased
triglyceride
levels
in
metabolic
syndrome
with
coronary
artery
disease.
Pak
J
Med
Sci
2010;
26:
667-672.
5.
Helvaci
MR,
Kaya
H,
Seyhanli
M,
Yalcin
A.
White
coat
hypertension
in
definition
of
metabolic
syndrome.
Int
Heart
J
2008;
49:
449-457.
6.
Helvaci
MR,
Kaya
H,
Seyhanli
M,
Cosar
E.
White
coat
hypertension
is
associated
with
a
greater
all-cause
mortality.
J
Health
Sci
2007;
53:
156-160.
7.
Helvaci
MR,
Kaya
H,
Duru
M,
Yalcin
A.
What
is
the
relationship
between
white
coat
hypertension
and
dyslipidemia?
Int
Heart
J
2008;
49:
87-93.
8.
Helvaci
MR,
Kaya
H,
Sevinc
A,
Camci
C.
Body
weight
and
white
coat
hypertension.
Pak
J
Med
Sci
2009;
25:
916-921.
9.
Helvaci
MR,
Aydogan
F,
Sevinc
A,
Camci
C,
Dilek
I.
Platelet
and
white
blood
cell
counts
in
severity
of
sickle
cell
diseases.
Pren
Med
Argent
2014;
100:
49-56.
10.
Helvaci
MR,
Sevinc
A,
Camci
C,
Keskin
A.
Atherosclerotic
background
of
cirrhosis
in
sickle
cell
patients.
Pren
Med
Argent
2014;
100:
127-133.
11.
Global
strategy
for
the
diagnosis,
management
and
prevention
of
chronic
obstructive
pulmonary
disease
2010.
Global
initiative
for
chronic
obstructive
lung
disease
(GOLD).
12.
Fisher
MR,
Forfia
PR,
Chamera
E,
Housten-Harris
T,
Champion
HC,
Girgis
RE,
et
al.
Accuracy
of
Doppler
echocardiography
in
the
hemodynamic
assessment
of
pulmonary
hypertension.
Am
J
Respir
Crit
Care
Med
2009;
179:
615-621.
13.
Schamroth
L.
Personal
experience.
S
Afr
Med
J
1976;
50:
297-300.
14.
Vandemergel
X,
Renneboog
B.
Prevalence,
aetiologies
and
significance
of
clubbing
in
a
department
of
general
internal
medicine.
Eur
J
Intern
Med
2008;
19:
325-329.
15.
Platt
OS,
Brambilla
DJ,
Rosse
WF,
Milner
PF,
Castro
O,
Steinberg
MH,
et
al.
Mortality
in
sickle
cell
disease.
Life
expectancy
and
risk
factors
for
early
death.
N
Engl
J
Med
1994;
330:
1639-1644.
16.
Helvaci
MR,
Aydin
Y,
Ayyildiz
O.
Hydroxyurea
may
prolong
survival
of
sickle
cell
patients
by
decreasing
frequency
of
painful
crises.
HealthMED
2013;
7:
2327-2332.
17.
Ekim
M,
Tumer
N,
Yalcinkaya
F,
Cakar
N.
Unilateral
renal
atrophy
and
hypertension
(imaging
techniques
in
children
with
hyperreninaemic
hypertension)
(a
case
report).
Int
Urol
Nephrol
1995;
27:
375-379.
18.
Simmons
MZ,
Wachsberg
RH,
Levine
CD,
Spiegel
N.
Intra-testicular
varicocele:
gray-scale
and
Doppler
ultrasound
findings.
J
Clin
Ultrasound
1996;
24:
371-374.
19.
Ozcan
H,
Aytac
S,
Yagci
C,
Turkolmez
K,
Kosar
A,
Erden
I.
Color
Doppler
ultrasonographic
findings
in
intratesticular
varicocele.
J
Clin
Ultrasound
1997;
25:
325-329.
20.
Mehta
AL,
Dogra
VS.
Intratesticular
varicocele.
J
Clin
Ultrasound
1998;
26:
49-51.
21.
O'Donnell
PG,
Dewbury
KC.
The
ultrasound
appearances
of
intratesticular
varicocele.
Br
J
Radiol
1998;
71:
324-325.
22.
Das
KM,
Prasad
K,
Szmigielski
W,
Noorani
N.
Intratesticular
varicocele:
evaluation
using
conventional
and
Doppler
sonography.
AJR
Am
J
Roentgenol
1999;
173:
1079-1083.
23.
Kurklinsky
AK,
Rooke
TW.
Nutcracker
phenomenon
and
nutcracker
syndrome.
Mayo
Clin
Proc
2010;
85:
552-559.
24.
Oteki
T,
Nagase
S,
Hirayama
A,
Sugimoto
H,
Hirayama
K,
Hattori
K,
et
al.
Nutcracker
syndrome
associated
with
severe
anemia
and
mild
proteinuria.
Clin
Nephrol
2004;
62:
62-65.
25.
Hilgard
P,
Oberholzer
K,
Meyer
zum
Büschenfelde
KH,
Hohenfellner
R,
Gerken
G.
The
"nutcracker
syndrome"
of
the
renal
vein
(superior
mesenteric
artery
syndrome)
as
the
cause
of
gastrointestinal
complaints.
Dtsch
Med
Wochenschr
1998;
123:
936-940.
26.
Little
AF,
Lavoipierre
AM.
Unusual
clinical
manifestations
of
the
Nutcracker
Syndrome.
Australas
Radiol
2002;
46:
197-200.
27.
Mohammadi
A,
Ghasemi-Rad
M,
Mladkova
N,
Masudi
S.
Varicocele
and
nutcracker
syndrome:
sonographic
findings.
J
Ultrasound
Med
2010;
29:
1153-1160.
|

