Table
1,
illustrates
the
SUMO1P3
expression
levels
and
demographic
characteristics
of
the
patients
including
age
and
gender.
Table
2,
shows
the
relationship
between
SUMO1P3
expression
levels
(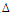 Ct)
in
GC
diagnosed
patients.
Ct)
in
GC
diagnosed
patients.
Figure
1,
gives
the
ROC
curve
of
the
SUMO1P3
levels
between
gastric
cancer
tissues
and
adjacent
non-tumor
tissues.
Table
1:
The
SUMO1P3
expression
levels
and
demographic
characteristics
of
the
patients
including
age
and
gender
Table
2:
The
relationship
between
SUMO1P3
expression
levels
( Ct)
in
GC
diagnosed
patients
Ct)
in
GC
diagnosed
patients
Figure
1:
The
ROC
curve
of
the
SUMO1P3
levels
between
gastric
cancer
tissues
and
adjacent
non-tumor
tissues

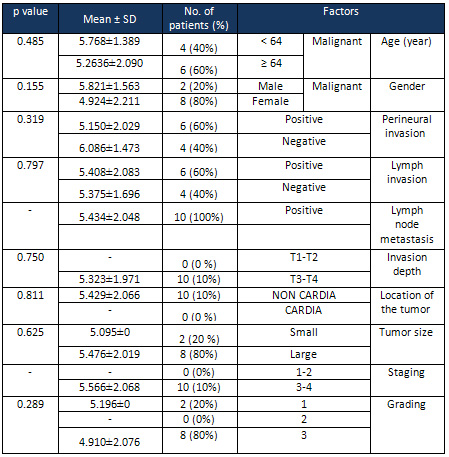
The
results
showed
that
SUMO1P3
levels
in
males
were
not
significantly
higher
than
those
in
females
(p
=
0.485,
Table
3).
No
significant
difference
of
SUMO1P3
expression
was
observed
between
patients
under
64
years
old
and
above
(p
=
0.155,
Table
3).
In
other
words,
patients
below
64
years-old
showed
higher
SUMO1P3
levels
compared
to
those
older
than
64.
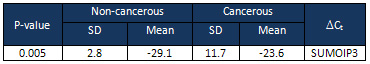
As
shown
in
Table
3,
the
SUMO1P3
levels
were
not
associated
with
perineural
invasion
(p
=
0.319),
lymphatic
invasion
(p
=
0.797),
invasion
depth
(p
=
0.790),
location
of
the
tumor
(p
=
0.811),
tumor
size
(p
=
0.635),
and
grading
(p
=
0.289).
Table
3:
The
relationship
between
SUMO1P3
expression
levels
( Ct)
and
pathological
factors
among
the
studied
patients.
Ct)
and
pathological
factors
among
the
studied
patients.
In
this
study,
we
were
interested
in
evaluating
the
expression
of
lncRNA
SUMO1P3
at
a
molecular
level
as
one
of
the
pseudogene-expressed
lncRNAs
in
GC
patients.
Recent
studies
have
shown
that,
lncRNA
plays
an
important
role
in
gastric
cancer
(9,
12).
However,
considering
the
pseudogene
expressed
lncRNAs,
the
potential
of
lncRNAs
as
a
clinical
diagnostic
marker
for
clinical
applications
is
still
basically
unknown.
Our
results
revealed
that
the
expression
levels
of
SUMO1P3,
one
of
the
transcripts
of
pseudogene,
were
not
up-regulated
in
gastric
cancer.
As
opposed
to
our
findings,
a
recent
publication
by
Mei
al
(6).
indicated
that
"pseudogenes
might
play
their
cancer-associated
roles
in
RNA
level".
We
also
followed
different
parameters
affecting
the
SUMO1P3
expression
in
our
patients
including;
age,
gender,
tumor
size,
differentiation,
lymphatic
metastasis,
invasion
(13,
14).
No
significant
up-regulation
of
SUMO1P3
expression
in
our
patients
with
GC
was
found
for
the
mentioned
factors
(Table
3).
We
found
that
SUMO1P3
expression
is
independent
of
age.
This
result
was
in
agreement
with
previous
reports,
stating
that
some
lncRNAs
such
as
gastric-cancer-associated
transcript
1,
GACAT1,
have
been
proved
to
be
independent
of
age
(9,
15,
16).
It
should
be
noted
that,
for
some
types
of
cancer,
gender
is
concerned
to
be
a
factor
to
influence
its
incidence
(9,
15,
16).
In
our
study,
we
investigated
that
gender
was
not
a
factor
that
is
significantly
related
to
SUMO1P3
expression
in
patients
with
GC
(p
=
0.485,
Table
3).
In
the
previously
published
papers,
the
relationship
between
invasion
and
lymphatic
metastasis
in
GC
and
miRNA
expression
has
been
reported
(17).
Our
results
indicated
a
non-significant
relationship
between
invasion
and
lymphatic
metastasis
in
GC
and
lncRNA
expression
(Table
3).
In
recent
years,
the
understanding
of
GC
biomarkers
has
undergone
a
marked
change
(1,
18-24).
Descriptions
of
gastric
wall
function
have
evolved
from
an
impermeable
and
passive
barrier
to
a
multifunctional
tissue
layer
with
an
active
role
in
dynamic
cellular
communication
and
adaptive
permeability
(1,
7,
25).
On
the
basis
of
the
present
results
and
according
to
the
used
method
for
our
patient
population,
we
can
believe
that
lncRNA
SUMO1P3
may
not
be
a
potential
biomarker
in
the
diagnosis
of
gastric
cancer.
However,
more
accurate
follow-up
studies
are
needed
for
the
evaluation
of
the
variations
of
lncRNA
SUMO1P3
expression
for
gastric
cancer
patients.
The
results
here
should
be
confirmed
in
larger
series,
considering
confounding
factors
(26,
27),
and
providing
a
more
detailed
assessment
of
lncRNA
SUMO1P3
levels
using
other
modalities.
In
this
work,
expression
of
lncRNA
SUMO1P3
in
gastric
cancer
patients
was
evaluated.
No
statistical
significant
change
of
pseudogene-expressed
lncRNA
SUMO1P3
was
seen
according
to
the
used
method
in
this
study.
Therefore,
pseudogene-expressed
lncRNA
SUMO1P3
may
not
be
a
potential
biomarker
in
the
diagnosis
of
gastric
cancer.
Acknowledgements
This
study
was
carried
out
as
a
PhD
thesis
by
HBGh
at
Shahid
Beheshty
University
of
Medical
Sciences,
Tehran,
Iran.
We
would
like
to
thank
the
staff
of
Dr
Baradaran
Pathology
Laboratory,
Isfahan
for
their
kind
contribution
to
this
study.
1.
Herszenyi
L,
Tulassay
Z.
Epidemiology
of
gastrointestinal
and
liver
tumors.
European
review
for
medical
and
pharmacological
sciences.
2010
Apr;14(4):249-58.
PubMed
PMID:
20496531.
2.
Zhang
EB,
Kong
R,
Yin
DD,
You
LH,
Sun
M,
Han
L,
et
al.
Long
noncoding
RNA
ANRIL
indicates
a
poor
prognosis
of
gastric
cancer
and
promotes
tumor
growth
by
epigenetically
silencing
of
miR-99a/miR-449a.
Oncotarget.
2014
Apr
30;5(8):2276-92.
PubMed
PMID:
24810364.
Pubmed
Central
PMCID:
4039162.
3.
Shahbazi-Gahrouei
D,
Keshtkar
M.
Magnetic
nanoparticles
and
cancer
treatment.
Immunopathol
Persa.
2016;2(1):e03.
4.
Catalano
V,
Labianca
R,
Beretta
GD,
Gatta
G,
de
Braud
F,
Van
Cutsem
E.
Gastric
cancer.
Critical
reviews
in
oncology/hematology.
2009
Aug;71(2):127-64.
PubMed
PMID:
19230702.
5.
Morabito
A,
Carillio
G,
Longo
R.
Systemic
treatment
of
gastric
cancer.
Critical
reviews
in
oncology/hematology.
2009
Jun;70(3):216-34.
PubMed
PMID:
18829344.
6.
Mei
D,
Song
H,
Wang
K,
Lou
Y,
Sun
W,
Liu
Z,
et
al.
Up-regulation
of
SUMO1
pseudogene
3
(SUMO1P3)
in
gastric
cancer
and
its
clinical
association.
Medical
oncology.
2013
Dec;30(4):709.
PubMed
PMID:
23996296.
7.
Li
CH,
Chen
Y.
Targeting
long
non-coding
RNAs
in
cancers:
progress
and
prospects.
The
international
journal
of
biochemistry
&
cell
biology.
2013
Aug;45(8):1895-910.
PubMed
PMID:
23748105.
8.
St
Laurent
G,
Wahlestedt
C,
Kapranov
P.
The
Landscape
of
long
noncoding
RNA
classification.
Trends
in
genetics
:
TIG.
2015
May;31(5):239-51.
PubMed
PMID:
25869999.
Pubmed
Central
PMCID:
4417002.
9.
Sun
W,
Wu
Y,
Yu
X,
Liu
Y,
Song
H,
Xia
T,
et
al.
Decreased
expression
of
long
noncoding
RNA
AC096655.1-002
in
gastric
cancer
and
its
clinical
significance.
Tumour
biology
:
the
journal
of
the
International
Society
for
Oncodevelopmental
Biology
and
Medicine.
2013
Oct;34(5):2697-701.
PubMed
PMID:
23645148.
10.
Wright
MW,
Bruford
EA.
Naming
'junk':
human
non-protein
coding
RNA
(ncRNA)
gene
nomenclature.
Human
genomics.
2011
Jan;5(2):90-8.
PubMed
PMID:
21296742.
Pubmed
Central
PMCID:
3051107.
11.
Yin
DD,
Liu
ZJ,
Zhang
E,
Kong
R,
Zhang
ZH,
Guo
RH.
Decreased
expression
of
long
noncoding
RNA
MEG3
affects
cell
proliferation
and
predicts
a
poor
prognosis
in
patients
with
colorectal
cancer.
Tumour
biology
:
the
journal
of
the
International
Society
for
Oncodevelopmental
Biology
and
Medicine.
2015
Jun;36(6):4851-9.
PubMed
PMID:
25636452.
12.
Xiao
B,
Guo
J.
Long
noncoding
RNA
AC096655.1-002
has
been
officially
named
as
gastric
cancer-associated
transcript
1,
GACAT1.
Tumour
biology
:
the
journal
of
the
International
Society
for
Oncodevelopmental
Biology
and
Medicine.
2013
Oct;34(5):3271.
PubMed
PMID:
23754450.
13.
Amiri
M.
On
the
occasion
of
world
cancer
day
2017;
breast
cancer.
J
Prev
Epidemiol.
2017;2(2):e07.
14.
Rastegari
F,
Rafieian-Kopaei
M.
Antioxidant
supplements
and
cancer.
Immunopathol
Persa.
2016;2(2):e14.
15.
Jemal
A,
Bray
F,
Center
MM,
Ferlay
J,
Ward
E,
Forman
D.
Global
cancer
statistics.
CA:
a
cancer
journal
for
clinicians.
2011
Mar-Apr;61(2):69-90.
PubMed
PMID:
21296855.
16.
Torre
LA,
Bray
F,
Siegel
RL,
Ferlay
J,
Lortet-Tieulent
J,
Jemal
A.
Global
cancer
statistics,
2012.
CA:
a
cancer
journal
for
clinicians.
2015
Mar;65(2):87-108.
PubMed
PMID:
25651787.
17.
Zheng
B,
Liang
L,
Huang
S,
Zha
R,
Liu
L,
Jia
D,
et
al.
MicroRNA-409
suppresses
tumour
cell
invasion
and
metastasis
by
directly
targeting
radixin
in
gastric
cancers.
Oncogene.
2012
Oct
18;31(42):4509-16.
PubMed
PMID:
22179828.
18.
Acharya
P,
Beckel
J,
Ruiz
WG,
Wang
E,
Rojas
R,
Birder
L,
et
al.
Distribution
of
the
tight
junction
proteins
ZO-1,
occludin,
and
claudin-4,
-8,
and
-12
in
bladder
epithelium.
American
journal
of
physiology
Renal
physiology.
2004
Aug;287(2):F305-18.
PubMed
PMID:
15068973.
19.
Al-Mamgani
A,
Heemsbergen
WD,
Peeters
ST,
Lebesque
JV.
Role
of
intensity-modulated
radiotherapy
in
reducing
toxicity
in
dose
escalation
for
localized
prostate
cancer.
International
journal
of
radiation
oncology,
biology,
physics.
2009
Mar
1;73(3):685-91.
PubMed
PMID:
18718725.
20.
Ataman
OU,
Barrett
A,
Davidson
S,
De
Haas-Kock
D,
Dische
S,
Dubray
B,
et
al.
Audit
of
effectiveness
of
routine
follow-up
clinics
after
radiotherapy
for
cancer:
a
report
of
the
REACT
working
group
of
ESTRO.
Radiotherapy
and
oncology
:
journal
of
the
European
Society
for
Therapeutic
Radiology
and
Oncology.
2004
Nov;73(2):237-49.
PubMed
PMID:
15542172.
21.
Budaus
L,
Bolla
M,
Bossi
A,
Cozzarini
C,
Crook
J,
Widmark
A,
et
al.
Functional
outcomes
and
complications
following
radiation
therapy
for
prostate
cancer:
a
critical
analysis
of
the
literature.
European
urology.
2012
Jan;61(1):112-27.
PubMed
PMID:
22001105.
22.
Amaral
PP,
Mattick
JS.
Noncoding
RNA
in
development.
Mammalian
genome
:
official
journal
of
the
International
Mammalian
Genome
Society.
2008
Aug;19(7-8):454-92.
PubMed
PMID:
18839252.
23.
Chern
CJ,
Beutler
E.
Biochemical
and
electrophoretic
studies
of
erythrocyte
pyridoxine
kinase
in
white
and
black
Americans.
American
journal
of
human
genetics.
1976
Jan;28(1):9-17.
PubMed
PMID:
2009.
Pubmed
Central
PMCID:
1684914.
24.
Fan
Y,
Wang
YF,
Su
HF,
Fang
N,
Zou
C,
Li
WF,
et
al.
Decreased
expression
of
the
long
noncoding
RNA
LINC00261
indicate
poor
prognosis
in
gastric
cancer
and
suppress
gastric
cancer
metastasis
by
affecting
the
epithelial-mesenchymal
transition.
Journal
of
hematology
&
oncology.
2016
Jul
21;9(1):57.
PubMed
PMID:
27439973.
Pubmed
Central
PMCID:
4955208.
25.
Huang
L,
Xu
A,
Li
T,
Han
W,
Wu
S,
Wang
Y.
Detection
of
perioperative
cancer
antigen
72-4
in
gastric
juice
pre-
and
post-distal
gastrectomy
and
its
significances.
Medical
oncology.
2013;30(3):651.
PubMed
PMID:
23820956.
26.
Nikzad
S,
Mahmoudi
G,
Amini
P,
Baradaran-Ghahfarokhi
M,
Vahdat-Moaddab
A,
Sharafi
SM,
et
al.
Effects
of
radiofrequency
radiation
in
the
presence
of
gold
nanoparticles
for
the
treatment
of
renal
cell
carcinoma.
Journal
of
renal
injury
prevention.
2017;6(2):103-8.
PubMed
PMID:
28497084.
Pubmed
Central
PMCID:
5423275.
27.
Nikzad
S.
The
effect
of
intermittent
radiotherapy
on
the
cells'
survival.
J
Radiobiol.
2015;2(1):11-5.
doi:
0.15171/jrb.2015.03.

