|
Safety of hydroxyurea in sickle
cell diseases
Mehmet
Rami Helvaci
(1)
Onder Tonyali (1)
Mustafa Yaprak (1)
Abdulrazak Abyad (2)
Lesley Pocock (3)
(1) Specialist of Internal Medicine, MD
(2) Middle-East Academy for Medicine of Aging,
MD
(3) medi+WORLD International
Correspondence:
Mehmet Rami Helvaci, MD
07400, ALANYA, Turkey
Phone: 00-90-506-4708759
Email: mramihelvaci@hotmail.com
Received: April 2019; Accepted: May 2019; Published:
June 1, 2019. Citation: Mehmet Rami Helvaci
et al. Safety of hydroxyurea in sickle cell
diseases. World Family Medicine. 2019;
17(6): 20-25. DOI: 10.5742MEWFM.2019.93656
|
Abstract
Background: We
tried to understand safety of hydroxyurea
in sickle cell diseases (SCDs).
Methods: The
study was performed between March 2007
and September 2013.
Results: The
study included 337 patients (169 females).
Mean number of painful crises per year
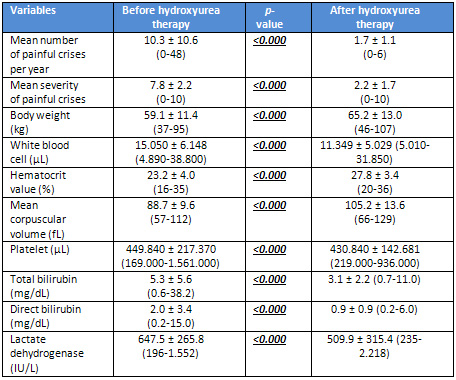
was decreased with hydroxyurea (10.3 versus
1.7 crises per year, p<0.000). Mean
severity of painful crises was decreased,
too (7.8/10 versus 2.2/10, p<0.001).
Although body weight, hematocrit (Hct)
value, and mean corpuscular volume (MCV)
increased, white blood cell (WBC) and
platelet (PLT) counts and direct bilirubin,
total bilirubin, and lactate dehydrogenase
(LDH) values of serum decreased (p<0.000
for all). We detected hepatotoxicity in
13 acute painful crises (two females and
11 males) among 1,211 episodes, totally
(1.0%). So it was significantly higher
in males (6.5% versus 1.1%, p<0.001).
All of them healed completely with withdrawal
of all of the medications but not hydroxyurea
alone. The solitary adverse effect of
hydroxyurea was bone marrow suppression
with prominent anemia in higher dosages.
It was detected in seven females (4.1%)
and nine males (5.3%, p>0.05), and
they completely healed with transient
withdrawal and decreased dosages thereafter.
Conclusion:
Hydroxyurea decreases frequency and severity
of painful crises, WBC and PLT counts,
direct and total bilirubin, and LDH values
of serum, whereas it increases body weight,
Hct value, and MCV. The rare (1.0%) and
reversible hepatotoxicity during acute
painful crises may not be related to hydroxyurea
alone, and the bone marrow suppression
with prominent anemia in higher dosages
may be the solitary adverse effect of
the drug.
Key words:
Hydroxyurea, sickle cell diseases, chronic
endothelial damage, atherosclerosis,
metabolic syndrome
|
Aging may be the major physical health problem
of the human being, and systemic atherosclerosis
may be the major underlying cause of it. Atherosclerosis
is an irreversible process mainly keeping afferent
vasculature due to the much higher blood pressure
(BP) in them. Accelerating factors of atherosclerosis
are collected under the heading of metabolic
syndrome including physical inactivity, smoking,
alcohol, chronic inflammation and infections,
cancers, excess weight, dyslipidemia, elevated
BP, and insulin resistance for the development
of irreversible diseases including obesity,
hypertension (HT), diabetes mellitus (DM), coronary
heart disease (CHD), chronic obstructive pulmonary
disease (COPD), cirrhosis, chronic renal disease
(CRD), peripheric artery disease, and stroke
(1-6). Early aging and premature death are the
terminal consequences of the syndrome. Similarly,
sickle cell diseases (SCDs) are systemic angiopathic
processes that are characterized by sickle-shaped
red blood cells (RBCs) caused by homozygous
inheritance of the hemoglobin S (Hb S) (7, 8).
Glutamic acid is replaced with a less polar
amino acid, valine, in the sixth position of
the beta chain of the Hb S. Presence of valine
promotes polymerisation of the Hb S. So Hb S
causes RBCs to change their normal elastic and
biconcave disc shaped structures to hard bodies.
The decreased elasticity of RBCs instead of
shapes may be the chief pathology of the diseases.
The sickling process is probably present in
the whole life span but exaggerated with several
stresses. RBCs can take their normal elastic
shapes after normalization of stresses of body,
but after repeated cycles of sickling and unsickling,
they become hard bodies, permanently. The hard
cells induced chronic endothelial damage together
with tissue ischemia and infarctions are the
final consequences of the diseases, so life
expectancy of such patients is decreased by
25 to 30 years (9). We tried to understand the
long-term safety of hydroxyurea therapy in patients
with the SCDs.
The study was performed in the Medical Facuty
of the Mustafa Kemal University between March
2007 and September 2013. All patients with SCDs
were enrolled into the study. SCDs are diagnosed
by the hemoglobin electrophoresis performed
via high performance liquid chromatography.
Their medical histories including smoking habit,
regular alcohol consumption, and leg ulcers
were learnt. Frequency of painful crises was
detected as a mean number of crises per year,
and severity of them as a mean degree between
0 to 10 according to patient’s self-explanation.
Cases with a history of three pack-year were
accepted as smokers, and cases with a history
of one drink a day for three years were accepted
as drinkers. A check up procedure including
body weight, serum creatinine value, hepatic
function tests, markers of hepatitis viruses
A, B, and C and human immunodeficiency virus,
an electrocardiography, a Doppler echocardiography,
an abdominal ultrasonography, a computed tomography
of brain, and a magnetic resonance imaging of
hips was performed. Other bone areas for avascular
necrosis were scanned according to the patients’
complaints. Cases with acute painful crisis
or any other inflammatory event were treated
at first, and then the spirometric pulmonary
function tests to diagnose COPD, the Doppler
echocardiography to measure the systolic BP
of pulmonary artery, and renal and hepatic function
tests were performed on the silent phase. The
criterion for diagnosis of COPD is post-bronchodilator
forced expiratory volume in 1 second/forced
vital capacity of less than 70% (10). Systolic
BP of the pulmonary artery of 40 mmHg or higher
during the silent phase is accepted as pulmonary
hypertension (11). CRD is diagnosed with a persistent
serum creatinine level of 1.3 mg/dL or higher
in males and 1.2 mg/dL or higher in females
on the silent phase. Cirrhosis is diagnosed
with physical examination findings, laboratory
parameters, ultrasonographic evaluation, and
liver biopsy in case of requirement. Digital
clubbing is diagnosed with the ratio of distal
phalangeal diameter to interphalangeal diameter
of greater than 1.0 and with the presence of
Schamroth’s sign (12, 13). A stress electrocardiography
was performed in cases with an abnormal electrocardiography
and/or angina pectoris. A coronary angiography
was obtained just for the stress electrocardiography
positive cases. So CHD was diagnosed either
angiographically or with the Doppler echocardiographic
findings as the movement disorders of the cardiac
walls. Then, a hydroxyurea therapy was initiated
to all patients with an initial dose of 15 mg/kg/day,
and then the dose was increased up to the final
dose of 35 mg/kg/day according to patients’
requirements and compliance. Finally, any adverse
effect of the therapy was followed up, and the
mean number and severity of painful crises,
mean body weight, white blood cell (WBC) and
platelet (PLT) counts, hematocrit (Hct) value,
mean corpuscular volume (MCV), and the direct
bilirubin, total bilirubin, and lactate dehydrogenase
(LDH) values of serum were compared before and
after the hydroxyurea therapy. Mann-Whitney
U test, Independent-Samples t test, and comparison
of proportions were used as the methods of statistical
analyses.
The
study
included
337
patients
with
the
SCDs
(169
females
and
168
males).
The
mean
ages
of
them
were
28.4
±
9.3
(8-59)
versus
29.8
±
9.3
(6-58)
years
in
females
and
males,
respectively
(p>0.05).
The
final
dose
of
35
mg/kg/day
with
hydroxyurea
therapy
was
just
achieved
in
25
cases
(7.4%),
and
the
usual
dose
was
500
mg
twice
daily
during
the
7-year
follow-up
period.
During
the
period,
the
mean
number
of
painful
crises
per
year
was
decreased
with
the
treatment,
significantly
(10.3
versus
1.7
crises
per
year,
p<0.000).
The
mean
severity
of
painful
crises
was
decreased,
too
(7.8/10
versus
2.2/10,
p<0.001).
Although
the
mean
body
weight,
mean
Hct
value,
and
MCV
increased,
the
WBC
and
PLT
counts
and
the
direct
bilirubin,
total
bilirubin,
and
LDH
values
of
serum
decreased
with
the
therapy,
significantly
(p<0.000
for
all)
(Table
1).
During
the
7-year
follow-up
period,
we
detected
hepatotoxicity
just
in
13
acute
painful
crises
among
1.211
episodes,
totally
(1.0%).
Interestingly,
two
of
the
patients
were
female
with
a
mean
age
of
38.5
years
and
11
cases
were
male
with
a
mean
age
of
32.3
years.
So
the
hepatotoxicity
during
acute
painful
crises
was
significantly
higher
in
males
(6.5%
versus
1.1%,
p<0.001).
All
of
the
cases
healed
completely
with
withdrawal
of
all
of
the
medications
but
not
hydroxyurea
alone.
The
solitary
adverse
effect
of
hydroxyurea
therapy
was
bone
marrow
suppression
with
prominent
anemia
in
higher
dosages
during
the
7-year
follow-up
period.
It
was
seen
in
seven
females
(4.1%)
with
a
mean
age
of
36.5
years
and
nine
males
with
a
mean
age
of
28.0
years
(5.3%,
p>0.05),
and
they
completely
healed
with
transient
withdrawal
and
decreased
dosages
of
hydroxyurea
thereafter.
Just
in
one
male
patient
with
an
age
of
22
years,
we
needed
to
support
with
two
units
of
RBCs
suspensions
due
to
the
symptomatic
palpitation.
None
of
the
patients
needed
any
supportive
therapy
for
thrombocytopenia
or
leukopenia.
Although
the
presence
of
prominent
anemia,
none
of
the
patients
was
on
acute
painful
crisis
during
the
detection.
On
the
other
hand,
we
detected
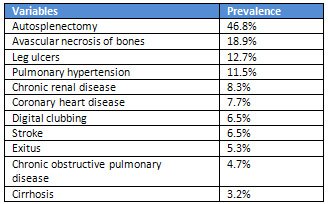
autosplenectomy
in
46.8%,
avascular
necrosis
of
bones
in
18.9%
(90.6%
at
the
hip
joints),
leg
ulcers
in
12.7%,
pulmonary
hypertension
in
11.5%,
CRD
in
8.3%,
CHD
in
7.7%,
digital
clubbing
in
6.5%,
stroke
in
6.5%,
exitus
in
5.3%,
COPD
in
4.7%,
and
cirrhosis
in
3.2%
of
the
patients
(Table
2).
Although
smoking
was
observed
in
6.5%
(22)
of
the
patients,
there
was
only
one
case
(0.2%)
of
regular
alcohol
consumption,
who
was
not
cirrhotic
at
the
moment.
Although
antiHCV
was
positive
in
two
of
the
cirrhotics,
HCV
RNA
was
detected
as
negative
by
polymerase
chain
reaction
in
both.
Prevalence
of
mortality
was
similar
in
both
genders
(4.7%
versus
5.9%
in
females
and
males,
respectively,
p>0.05),
and
mean
ages
of
such
cases
were
32.1
versus
29.1
years
in
females
and
males,
respectively
(p>0.05).
Table
1:
Characteristic
features
of
sickle
cell
patients
before
and
after
hydroxyurea
therapy
Table
2:
Sickle
cell
patients
with
associated
disorders
|

