|
Rota virus vaccine- induced
intussusception: A case report study
Mohammad
M. Alkot (1)
Hossam S Abdelbaki (2)
Mohammad S. Al-Fageah (3)
Ebtesam A. Al-Sulami (3)
(1) Family medicine department , Menoufia university,
Egypt
(2) Pediatric department , Menoufia university,
Egypt
(3) Faculty of medicine , Umm Al-Qura university
Correspondence:
Mohammad M. Alkot
Family medicine department,
Menoufia university, Egypt
Email: mohammed_elkott@yahoo.com
|
Abstract
Introduction:
Intussusception is a rare potential adverse
effect of oral rotavirus vaccination,
estimated to occur in approximately 1:100,000
vaccine recipients.
Case presentation: Six-months old
boy presented with vomiting for 3 days,
colicky abdominal pain, and did not pass
stool for one day prior to the admission.
Passage of reddish soft jelly like motion
was reported by his mother. No seizure,
no cough, no jaundice, no skin/joint/
bone complications. History of similar
condition 2 months ago at age of 4 months
(one week following his scheduled vaccination
which contains Rota vaccine).Physical
examination; lethargic, afebrile with
stable vital signs, abdomen was soft,
lax with no distension or palpable mass.
Per rectal (PR) examination was blood
stained. He was diagnosed with intussusception.
Hydrostatic reduction was failed. Laparotomy
resection of 6 CM of terminal ileum 15CM
away from ileocaecal valve with appendectomy.
Patient underwent uneventful postoperative
course and discharged in good condition.
Conclusion: Although the reported
vaccine-induced intussusception occurs
every now and then, the overall risk benefit
balance of vaccines remains positive So
World Health Organization (WHO) and the
Australian Technical Advisory Group on
Immunization (ATAGI) have recommended
the continued use of rotavirus vaccine
for infants as it reduces annual hospital
admissions in children under 5 years due
to rotavirus gastroenteritis.
Key words: Intussusception,
Rota virus, Vaccination
|
Rotavirus is the leading cause of severe diarrhea
in infants and children worldwide, leading to
more than half a million deaths each year in
children under the age of five years. The first
rotavirus vaccine, Rotashield, was introduced
in 1999. It was voluntarily withdrawn from the
market within a year because post-marketing
surveillance found 1-2 excess cases of intussusception
per 10,000 recipients [1]. Two newer vaccines,
Rotateq and Rotarix, were thought not to carry
that risk, but two new trials have shown that
they do. Still, the risk is small and the benefits
of the vaccines are great. Newer vaccines, Rotateq
and Rotarix, were licensed only after testing
(in over 60,000 infants for each) failed to
find any association with intussusception [2].
Those trials were designed to have enough statistical
power to detect a risk similar to that of RotaShield.
Both new vaccines contain live, attenuated strains
of the virus and are given orally. Rotateq is
a pentavalent (prepared from 5 strains) vaccine
given in 3 doses at age 2, 4, and 6 months.
Rotarix is monovalent (prepared from 1 strain)
and is given in 2 doses at age 2 and 4 months.
Either is recommended, but about 10 times more
doses of the pentavalent vaccine have been administered.
After the new vaccines came into common use,
studies in other countries pointed to a small
increase in intussusception with the newer vaccines,
but still at a much lower rate than with Rotashield[3].
Intussusceptions after administration of Rota
vaccine is a very rare serious complication
that could be easily missed. Intussusception
is a "telescoping" of the intestine
where one section slides inside another section.
This can cut off the blood supply, block the
intestine, and cause tears, infections, and
death. Most cases are in young children [4].
The baseline incidence of intussusception in
children is 1-4 per 1,000. Most cases have no
identified cause, but the most plausible candidate
is hypertrophied lymphoid tissue resulting from
viral illnesses, especially rotavirus infections.
They have severe abdominal pain (intermittent
at first), and pass blood in the stool, typically
mixed with mucus and having the appearance of
currant jelly [5]. A barium enema can confirm
the diagnosis and simultaneously treat it. Sometimes
surgery is needed[6].
Six months old boy presented to emergency
room complaining of vomiting for 3 days, colicky
abdominal pain, and he did not pass stool for
1 day prior to the admission. The patient developed
fever and decreased feeding. He developed vomiting
of large amount of undigested food, non- bilious
passage of reddish soft jelly like motion as
reported by the mother. He suffered from decreased
activates. No seizure, no cough, no jaundice,
no skin/joint/bone complication. History of
similar condition 2 months ago at the age of
4 months (one week following his scheduled vaccination
which contains Rota vaccine). On physical examination,
he looks lethargic afebrile with stable vital
signs, abdomen was soft, lax, not distended
with no palpable mass. Per rectal (PR) examination
was blood stained.
Complete
blood
picture
showed
:
Hemoglobin
11.3g/dl,
White
Blood
Ccll
count
21.5/cc,
Platelet
count
367/cc
Chemistry
and
coagulation
profile
results
were
within
normal.
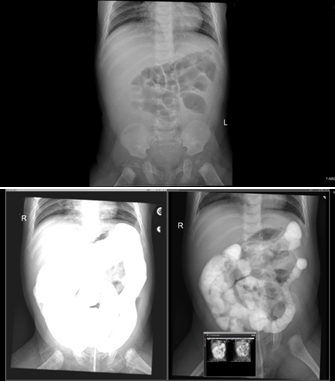
Radiology;
A-P
erect
X-ray
film
showed
dilated
bowl
loops.
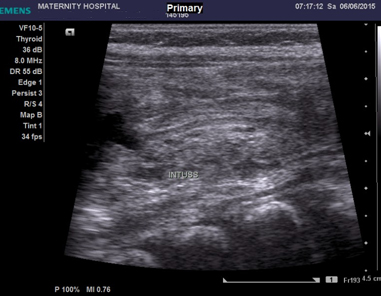
Abdominal
ultrasound
(U/S)
showed
intussusception
with
minimal
free
fluid.
Figure
1:
A-P
erect
X-Ray
film
showed:
Dilated
bowl
loops
Figure
2:
Abdominal
ultrasound
showed
intussusception
with
minimal
free
fluid
Hydrostatic
reduction
was
done
initially
and
confirmed
by
contrast
enema.
After
12
hours,
he
developed
vomiting
and
redcurrant
jelly
stool.
Repeated
abdomen
ultrasound
showed
ileo-ileal
intussusception
with
failed
hydrostatic
reduction.
Laparotomy
proceeded
and
revealed
ileo-ileal
intussusception
with
intraluminal
polyp.
Resection
of
6
Cm
of
terminal
ileum
15Cm
away
from
ileocaecal
valve
with
appendectomy
was
carried
out.
The
patient
underwent
uneventful
postoperative
course
and
was
discharged
in
good
condition.
Risk
with
RRV-TV:
In
1999,
just
over
a
year
after
human-rhesus
rotavirus
reassortant
vaccine
(RRV-TV,
RotaShield)
was
licensed,
it
was
withdrawn
from
the
market
because
of
an
epidemiologic
link
to
intussusception[4].
The
increased
risk
was
estimated
to
be
approximately
22-fold
over
the
background
risk
within
five
to
seven
days
of
vaccination
and
overall
approximately
one
excess
case
for
every
10,000
to
12,000
vaccinated
infants
[5,6].
The
mechanism
of
this
association
is
unclear.
One
hypothesis
is
that;
vaccination
triggered
intussusception
in
infants
who
were
likely
to
develop
intussusception
with
any
enteric
infection,
based
upon
the
observation
that
rates
of
intussusception
were
actually
lower
among
vaccine
recipients
than
non-vaccinees
in
the
period
of
4
to
12
weeks
after
vaccination
[7]
.Thus,
RRV-TV
may
have
caused
intussusception
in
infants
who
otherwise
would
not
have
experienced
intussusception,
but
it
also
may
have
protected
against
natural
rotavirus
infection-induced
intussusception
in
others.
Risk
with
RV5
and
RV1:
Intussusception
is
a
rare
potential
adverse
effect
of
oral
rotavirus
vaccination,
estimated
to
occur
in
approximately
1
in
20,000
to
1
in
100,000
vaccine
recipients
[8-13].
A
history
of
intussusception
is
a
contraindication
to
rotavirus
vaccination
[14,15],
but
for
infants
without
a
history
of
intussusception,
the
risk
of
intussusception
after
rotavirus
vaccination
is
much
lower
than
the
risk
of
severe
rotavirus
gastroenteritis
in
children
who
do
not
receive
rotavirus
vaccine
[16-18].
Parents
should
contact
their
child's
healthcare
provider
if
the
child
develops
signs
of
intussusception
(ie,
stomach
pain,
vomiting,
diarrhea,
blood
in
the
stool,
or
change
in
bowel
habits)
at
any
time
after
vaccination,
especially
within
the
first
14
days
after
a
dose
was
given.
Pre-licensure
studies
of
pentavalent
human-bovine
rotavirus
reassortant
vaccine
(RV5)
and
attenuated
human
rotavirus
vaccine
(RV1),
found
no
increased
risk
of
intussusception
among
vaccine
recipients
compared
with
placebo
recipients
[19.20],
however,
post-licensure
studies
conducted
by
the
Centers
for
Disease
Control
and
Prevention
(CDC),
the
Vaccine
Safety
Data
link
investigation
group,
the
US
Food
and
Drug
Administration
(Post-licensure
Rapid
Immunization
Safety
Monitoring),
vaccine
manufacturers,
and
others
suggest
a
rare
association
between
RV5
and
RV1
vaccination
and
intussusception
within
21
days
of
the
first
dose
[8-13,21].
The
absolute
number
of
estimated
rotavirus
hospitalizations
prevented
by
rotavirus
vaccines
far
exceeds
that
of
cases
of
intussusception
associated
with
rotavirus
vaccine
(eg,
65,000
hospitalizations
prevented
and
40
to
120
cases
of
intussusception
per
year
in
the
United
States)
[16].
The
CDC
continues
to
recommend
universal
rotavirus
vaccination
for
infants
in
the
United
States.
A
rotavirus
vaccine
(either
one)
is
recommended
by
the
CDC,
the
American
Pediatric
Association,
and
other
professional
groups
as
a
part
of
the
routine
immunization
schedule
in
the
United
States.
Parents
should
be
informed
of
the
signs
of
intussusception
and
should
monitor
their
infants
especially
in
the
first
7
days
after
vaccination;
and
since
intussusception
can
recur,
caution
is
advised
in
children
who
have
a
history
of
intussusception.
1-
Peter
G,
Myers
MG,
National
Vaccine
Advisory
Committee,
National
Vaccine
Program
Office.
Intussusception,
rotavirus,
and
oral
vaccines:
summary
of
a
workshop.
Pediatrics.
2002;110(6):e67.
2-
Centers
for
Disease
Control
and
Prevention
(CDC).
Intussusception
among
recipients
of
rotavirus
vaccine--United
States,
1998-1999.
MMWR
Morb
Mortal
Wkly
Rep.
1999;48(27):577.
3-
Murphy
TV,
Gargiullo
PM,
Massoudi
MS,
Nelson
DB,
Jumaan
AO,
Okoro
CA,
Zanardi
LR,
Setia
S,
Fair
E,
LeBaron
CW,
Wharton
M,
Livengood
JR,
Rotavirus
Intussusception
Investigation
Team.
Intussusception
among
infants
given
an
oral
rotavirus
vaccine.
N
Engl
J
Med.
2001;344(8):564.
4-
Murphy
BR,
Morens
DM,
Simonsen
L,
Chanock
RM,
La
Montagne
JR,
Kapikian
AZ.
Reappraisal
of
the
association
of
intussusception
with
the
licensed
live
rotavirus
vaccine
challenges
initial
conclusions.
J
Infect
Dis.
2003;187(8):1301.
5-
Clark
A,
Jit
M,
Andrews
N,
Atchison
C,
Edmunds
WJ,
Sanderson
C.
Evaluating
the
potential
risks
and
benefits
of
infant
rotavirus
vaccination
in
England.
Vaccine
2014
Jun
17;32(29):3604-10.
doi:
10.1016/j.vaccine.2014.04.082.
Epub
2014
May
9.
6-
Glass
RI,
Parashar
UD.
Rotavirus
vaccines--balancing
intussusception
risks
and
health
benefits.
N
Engl
J
Med.
2014;370(6):568.
7-
Murphy
BR,
Morens
DM,
Simonsen
L,
Chanock
RM,
La
Montagne
JR,
Kapikian
AZ.
Reappraisal
of
the
association
of
intussusception
with
the
licensed
live
rotavirus
vaccine
challenges
initial
conclusions.
J
Infect
Dis.
2003;187(8):1301.
8-
Anderson
EJ,
Shippee
DB,
Weinrobe
MH,
Davila
MD,
Katz
BZ,
Reddy
S,
Cuyugan
MG,
Lee
SY,
Simons
YM,
Yogev
R,
Noskin
GA.
Indirect
protection
of
adults
from
rotavirus
by
pediatric
rotavirus
vaccination.
Clin
Infect
Dis.
2013;56(6):755.
9-
Gastañaduy
PA,
Curns
AT,
Parashar
UD,
Lopman
BA.
Gastroenteritis
hospitalizations
in
older
children
and
adults
in
the
United
States
before
and
after
implementation
of
infant
rotavirus
vaccination.
JAMA.
2013
Aug;310(8):851-3.
10-
Cortese
MM,
Dahl
RM,
Curns
AT,
Parashar
UD.
Protection
against
gastroenteritis
in
US
households
with
children
who
received
rotavirus
vaccine.
J
Infect
Dis.
2015;211(4):558.
11-
Mast
TC,
Wang
FT,
Su
S,
Seeger
JD.
Evidence
of
herd
immunity
and
sustained
impact
of
rotavirus
vaccination
on
the
reduction
of
rotavirus-related
medical
encounters
among
infants
from
2006
through
2011
in
the
United
States.
Pediatr
Infect
Dis
J.
2015;34(6):615.
12-
Patel
MM,
López-Collada
VR,
Bulhões
MM,
De
Oliveira
LH,
Bautista
Márquez
A,
Flannery
B,
et.al.,
Intussusception
risk
and
health
benefits
of
rotavirus
vaccination
in
Mexico
and
Brazil.
N
Engl
J
Med.
2011;364(24):2283.
13-
Velázquez
FR,
Colindres
RE,
Grajales
C,
Hernández
MT,
Mercadillo
MG,
Torres
FJ,
Cervantes-Apolinar
M,
DeAntonio-Suarez
R,
Ortega-Barria
E,
Blum
M,
Breuer
T,
Verstraeten
T.
Postmarketing
surveillance
of
intussusception
following
mass
introduction
of
the
attenuated
human
rotavirus
vaccine
in
Mexico.
Pediatr
Infect
Dis
J.
2012;31(7):736.
14-
Centers
for
Disease
Control
and
Prevention
(CDC).
Addition
of
history
of
intussusception
as
a
contraindication
for
rotavirus
vaccination.
MMWR
Morb
Mortal
Wkly
Rep.
2011;60(41):1427.
15-
Haber
P,
Patel
M,
Pan
Y,
Baggs
J,
Haber
M,
Museru
O,
Yue
X,
Lewis
P,
Destefano
F,
Parashar
UD.
Intussusception
after
rotavirus
vaccines
reported
to
US
VAERS,
2006-2012.
Pediatrics.
2013
Jun;131(6):1042-9.
Epub
2013
May
13.
16-
Carlin
JB,
Macartney
KK,
Lee
KJ,
Quinn
HE,
Buttery
J,
Lopert
R,
Bines
J,
McIntyre
PB.
Intussusception
risk
and
disease
prevention
associated
with
rotavirus
vaccines
in
Australia's
National
Immunization
Program.
Clin
Infect
Dis.
2013;57(10):1427.
17-
Weintraub
ES,
Baggs
J,
Duffy
J,
Vellozzi
C,
Belongia
EA,
Irving
S,
Klein
NP,
Glanz
JM,
Jacobsen
SJ,
Naleway
A,
Jackson
LA,
DeStefano
F.
Risk
of
intussusception
after
monovalent
rotavirus
vaccination.
N
Engl
J
Med.
2014;370(6):513.
18-
Yih
WK,
Lieu
TA,
Kulldorff
M,
Martin
D,
McMahill-Walraven
CN,
Platt
R,
Selvam
N,
Selvan
M,
Lee
GM,
Nguyen
M.
Intussusception
risk
after
rotavirus
vaccination
in
U.S.
infants.
N
Engl
J
Med.
2014;370(6):503.
19-
Vesikari
T,
Matson
DO,
Dennehy
P,
Van
Damme
P,
Santosham
M,
Rodriguez
Z,
Dallas
MJ,
Heyse
JF,
Goveia
MG,
Black
SB,
Shinefield
HR,
Christie
CD,
Ylitalo
S,
Itzler
RF,
Coia
ML,
Onorato
MT,
Adeyi
BA,
Marshall
GS,
Gothefors
L,
Campens
D,
Karvonen
A,
Watt
JP,
O'Brien
KL,
DiNubile
MJ,
Clark
HF,
Boslego
JW,
Offit
PA,
Heaton
PM,
Rotavirus
Efficacy
and
Safety
Trial
(REST)
Study
Team.
Safety
and
efficacy
of
a
pentavalent
human-bovine
(WC3)
reassortant
rotavirus
vaccine.
N
Engl
J
Med.
2006;354(1):23.
20-
Ruiz-Palacios
GM,
Pérez-Schael
I,
Velázquez
FR,
Abate
H,
Breuer
T,
Clemens
SC,
et
al.,
Human
Rotavirus
Vaccine
Study
Group.
Safety
and
efficacy
of
an
attenuated
vaccine
against
severe
rotavirus
gastroenteritis.
N
Engl
J
Med.
2006;354(1):11.
21-
Desai
R,
Cortese
MM,
Meltzer
MI,
Shankar
M,
Tate
JE,
Yen
C,
Patel
MM,
Parashar
UD.
Potential
intussusception
risk
versus
benefits
of
rotavirus
vaccination
in
the
United
States.
Pediatr
Infect
Dis
J.
2013
Jan;32(1):1-7.
|

