|
|
 |
| ............................................................. |
|
|
| ........................................................ |
| From
the Editor |

|
Editorial
A. Abyad (Chief Editor) |
|
|
|
|
........................................................
|
Original
Contribution/Clinical Investigation
|
|

|
<-- Turkey -->
Very high
levels of C-reactive protein should alert the
clinician to the development of acute chest
syndrome in sickle cell patients
[pdf version]
Can Acipayam, Sadik Kaya, Mehmet Rami Helvaci,
Gül Ilhan, Gönül Oktay
<-- Jordan -->
Seroprevalence
of HBV, HCV, HIV and syphilis infections among
blood donors at Blood Bank of King Hussein Medical
Center: A 3 Year Study
[pdf
version]
Baheieh Al Abaddi, Maha Al Amr, Lamees Abasi,
Abeer Saleem, Nisreen Abu hazeem, Ahmd Marafi
|
|
........................................................ |
Medicine and Society
........................................................
International Health
Affairs
.......................................................
Education
and Training
.......................................................
Continuing
Medical Education
|
Chief
Editor -
Abdulrazak
Abyad
MD, MPH, MBA, AGSF, AFCHSE
.........................................................
Editorial
Office -
Abyad Medical Center & Middle East Longevity
Institute
Azmi Street, Abdo Center,
PO BOX 618
Tripoli, Lebanon
Phone: (961) 6-443684
Fax: (961) 6-443685
Email:
aabyad@cyberia.net.lb
.........................................................
Publisher
-
Lesley
Pocock
medi+WORLD International
11 Colston Avenue,
Sherbrooke 3789
AUSTRALIA
Phone: +61 (3) 9005 9847
Fax: +61 (3) 9012 5857
Email:
lesleypocock@mediworld.com.au
.........................................................
Editorial
Enquiries -
abyad@cyberia.net.lb
.........................................................
Advertising
Enquiries -
lesleypocock@mediworld.com.au
.........................................................
While all
efforts have been made to ensure the accuracy
of the information in this journal, opinions
expressed are those of the authors and do not
necessarily reflect the views of The Publishers,
Editor or the Editorial Board. The publishers,
Editor and Editorial Board cannot be held responsible
for errors or any consequences arising from
the use of information contained in this journal;
or the views and opinions expressed. Publication
of any advertisements does not constitute any
endorsement by the Publishers and Editors of
the product advertised.
The contents
of this journal are copyright. Apart from any
fair dealing for purposes of private study,
research, criticism or review, as permitted
under the Australian Copyright Act, no part
of this program may be reproduced without the
permission of the publisher.
|
|
|
| August 2014 -
Volume 12 Issue 6 |
|
Very
high levels of C-reactive protein should alert
the clinician to the development of acute chest
syndrome in sickle cell patients
Can Acipayam (1)
Sadik Kaya (2)
Mehmet Rami Helvaci (3)
Gül Ilhan (4)
Gönül Oktay (5)
(1)
Department of Pediatric Hematology and Oncology,
Medical Faculty of Mustafa Kemal University,
Hatay, Turkey.
(2) Department of Pediatric, Hatay Antakya State
Hospital, Hatay, Turkey.
(3) Department of Internal Medicine, Medical
Faculty of Mustafa Kemal University, Hatay,
Turkey.
(4) Department of Hematology, Hatay Antakya
State Hospital, Hatay, Turkey.
(5) Hemoglobinopathy Center, Hatay Antakya State
Hospital, Hatay, Turkey.
Correspondence:
Can Acipayam, MD
Department of Pediatric Hematology and Oncology
Mustafa Kemal University, Tayfur Ata Sokmen
Medical School,
Serinyol, Hatay 31000, Turkey.
Phone Number: +90 326 229 10 00
Fax Number: +90 326 245 56 54
Email:
cacipayam@hotmail.com
|
Abstract
Purpose:
Acute chest syndrome (ACS) is associated
with both inflammation and tissue ischemia.
C-reactive protein (CRP) is a marker of
systemic inflammation. The aim of this
study was to determine if a relationship
exists between CRP and severe ACS.
Methods: Forty-three patients with
painful crises (range: 4-18 years, mean:
11.4 years) hospitalized between 2012
and 2014, consisting of 23 patients with
ACS and 20 patients without ACS (uncomplicated
vaso-occlusive crisis) were recruited
into this study. Retrospective data were
obtained directly from inpatient medical
records. ACS was defined as a new pulmonary
infiltrate on chest radiograph after admission
and before discharge. CRP was measured
using a BN II Nephelometer.
Results: Mean length of hospital
stay of ACS patients was 9.9 days (range
7-18 days) while that of patients without
ACS was 5.2 days (range 2-10 days), (p=0.001).
In 91% of the ACS cases, ACS developed
within the first 72 hours, while the remaining
9% cases were admitted for vaso-occlusive
crises but subsequently developed ACS
during their hospital stay on the 5th
to 7th days. CRP levels on admission were
significantly higher in patients with
ACS than those without ACS (p=0.001).
Conclusion: We investigated CRP
in relation to ACS in children with sickle
cell disease (SCD). Elevated CRP was determined
in all ACS patients with SCD. CRP may
be a superior diagnostic marker and herald
severe ACS in individuals with SCD.
Key words:
Sickle cell diseases, Acute chest syndrome,
C-reactive protein
|
Acute chest syndrome (ACS) is a frequent complication
of sickle cell disease (SCD). ACS represents grounds
for hospital admission and is the most common
cause of death in patients with SCD. ACS is defined
as a new pulmonary infiltrate and some combination
of fever, chest pain and signs and symptoms of
pulmonary diseases, such as tachypnea, cough and
dyspnea (1-3).
There are many causes of ACS, and the pathogenesis
is complex and not thoroughly understood. The
trigger for ACS in an individual patient generally
cannot be identified. Although infection is the
most common identifiable cause of ACS, there are
other important triggers including vaso-occlusive
crisis (VOC), rib infarction, bone marrow infarction,
fat embolism and asthma. The presenting signs
and symptoms of ACS can be highly variable and
affected individuals may have a normal initial
physical examination. ACS often develops in the
setting of a vaso-occlusive episode or with other
acute manifestations of SCD, frequently after
two to three days of severe vaso-occlusive pain.
ACS can progress rapidly (over several hours to
days) to requiring intubation and mechanical ventilatory
support (3-5).
Acute phase proteins such as C-reactive protein
(CRP) are well recognized for their applications
in human diagnostic medicine and are reported
to be valuable in the diagnosis and prognosis
of cardiovascular disease, SCD, autoimmunity,
organ transplant, and cancer treatment. CRP can
be used together with signs and symptoms and other
tests to evaluate an individual for acute or chronic
inflammatory conditions. Previous studies have
reported a strong association between increased
CRP levels and VOC. The elevated CRP in SCD may
be in response to endothelial damage due to the
blockage of the vascular endothelium by sickled
erythrocytes (6,7).
As the sickle cell painful crisis is associated
with both inflammation and tissue ischemia, we
hypothesized that serum CRP levels may rise during
and in association with severe ACS. The aim of
this study was to evaluate CRP levels in children
with SCD in ACS and during VOC.
We retrospectively reviewed the medical records
of patients under 18 years and admitted for VOC
between 2012 and 2014. Patients' data were obtained
directly from inpatient medical records and from
the hospital-based computer system accessed by
the same physician. Data collected included demographic
information such as gender and date of birth.
Other variables included dates of admission and
discharge. Medical charts for all patients were
reviewed for data concerning the chief complaint,
respiratory symptoms, fever, peripheral blood
white blood cell (WBC) count, biochemical tests
(blood urea nitrogen (BUN), creatinine and CRP),
chest radiograph and chest computed tomography
(CT) reports, receipt of blood transfusion and
erythrocytapheresis, painful time, admission to
hospital, duration of hospitalization, discharge
diagnosis, mortality and complications during
the study. The final discharge diagnosis of ACS
was defined as a new pulmonary infiltrate on chest
radiograph after admission and before discharge.
ACS was recorded according to the current criteria:
new infiltrate visible at chest X-ray (involving
at least one complete lung segment consistent
with the presence of alveolar consolidation) associated
with one or more symptoms, such as fever, cough,
tachypnea, breathing difficulties or new-onset
hypoxia (8).
Blood samples were obtained during visits to the
outpatient clinic or at presentation to the emergency
department for a painful crisis. Standard blood
counts were performed in EDTA-anti-coagulated
blood (Sysmex XT- 2000i, USA). Biochemical investigation
was performed with a Modular Analytics P800 analyzer
(Roche Diagnostics, Indianapolis, IN) using spectrophotometric
methods. We measured the inflammatory biomarker
CRP in all patients using a BN II Nephelometer.
Serum CRP values were considered normal between
0 and 5 mg/dl. Patients were divided into two
groups, with ACS and without ACS. Patients without
ACS were selected from the VOC group without complications.
Statistical analysis was performed on SSPS for
Windows version 15 (SPSS Inc., Chicago, IL, USA).
Numerical data were expressed as mean±standard
deviation (SD), mean, maximum and minimum. For
data analysis, patients were divided into two
groups, with ACS and without. The Mann-Whitney
U-test and chi-square test were used for comparison
between the two groups. The chi-square test was
used to evaluate qualitative variables, while
the Mann-Whitney U-test was used to examine relations
between non-parametric data. p<0.05 was considered
significant.
Table 1: Demographic and clinical characteristics
of children with SCD admitted due to an initial
painful episode over a 2-year period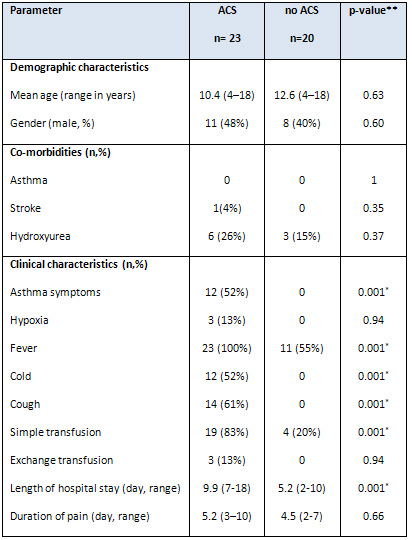
*Statistically significant at p < 0.05. ACS:
Acute chest syndrome. **p-values were calculated
using the chi-square and Mann-Whitney U tests.
Table 2: Hematological and biochemical parameters
of patients with or without ACS
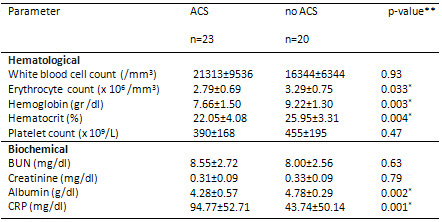
Data are arithmetical means ± SD. * Statistically
significant at p < 0.05. ACS: Acute chest
syndrome, BUN; blood urea nitrogen, CRP; C-reactive
protein. **p-values were calculated using the
Mann-Whitney U test.
A
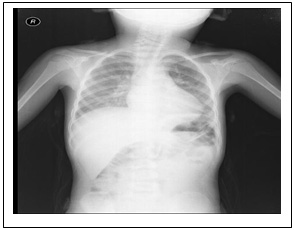
B
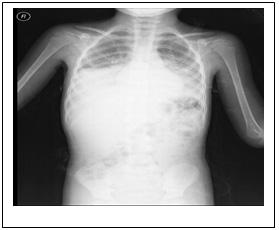
C
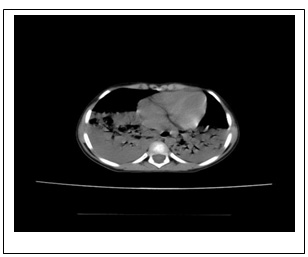
D
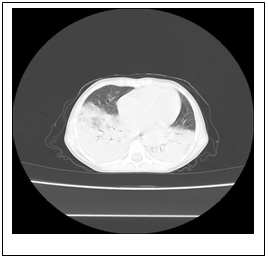
Figure 1: Chest X-ray and chest computed
tomography (CT) image. At admission, chest X-ray
revealed normal. (B) Within 48 hours, chest
X-ray showed development of a new bilateral
pulmonary infiltrate. Axial view of chest CT
using mediastinal (C) and parenchymal (D) windows
showed extensive bilateral pulmonary infiltrates
and lobar consolidation (involvement of bilateral
lung).
Serum CRP levels in patients with ACS were comparable
to those in patients without, but increased significantly
during the disease. ACS has a multifactorial etiology,
including a variety of inciting events that trigger
deoxygenation of HbS, leading to its polymerization
and to red blood cell sickling with subsequent
vaso-occlusion, ischemia, and endothelial dysfunction
(9,10). Heightened proinflammatory cytokine production
has been reported in individuals with SCA during
VOC (7). Elevated levels of CRP, a general marker
of inflammation, have previously been reported
in ACS patients with SCD (11-14).
Previous studies from our institution have shown
that serum CRP levels increase markedly in SCD
patients with ACS and that sequential measurements
of CRP are useful in predicting the subsequent
development of ACS in patients hospitalized for
VOC (11-14). This study confirms the earlier findings
of increased CRP levels in patients with ACS.
In contrast to other studies, CRP levels in our
ACS group were significantly higher than those
in the non-ACS group. Patients with SCD and elevated
CRP levels must be closely monitored for development
of ACS. Chest pain was present in 15 of the 23
patients with ACS at time of presentation and
back pain in 8. One patient with chest pain developed
intense abdominal pain in the right upper quadrant
on the 2nd day of hospitalization. Consolidation
and effusion was determined in the right lower
lobe at abdominal USG and chest X-ray.
Diagnosis of ACS can be difficult at times and
depends on the experience of the physician. The
clinical symptoms described above should alert
the physician to the possibility of ACS. Physical
examination may reveal tachypnea, dyspnea, hypoxia,
decreased air entry, wheezes, and rales. Physical
examination alone can, at times, be unreliable
in the diagnosis of ACS, and up to 60% of cases
are missed by clinicians without radiological
confirmation. Additionally, because pain crises
often herald ACS, a chest radiograph may be indicated
in patients hospitalized for pain, particularly
when they develop fever and/or respiratory symptoms.
It should be noted, though, that lung infiltrates
may not appear in radiographs before 48 to 72
hours after onset of clinical symptoms (9). Vichinsky
et al. (15) described the development of ACS within
a mean 2.5 days after admission for pain. This
parallels an earlier report by Bellet et al. (16)
which determined abnormal chest X-ray 2.4 days
after admission for pain. In agreement with previous
studies, ACS developed within the first 72 hours
in 21 of the 23 patients with ACS in our study.
Chest X-rays were performed on all patients with
clinical systems involving the respiratory system
and widespread lobar consolidation was observed
in all. Chest CT was performed on all patients
in terms of effusion and complications. In agreement
with Abbas et al.'s study (9), pulmonary infiltrates
were determined on radiographs within the first
72 hours after onset of clinical symptoms in 91%
of patients. Morris et al. (17) also reported
the unreliability of physical examination in the
detection of ACS in febrile patients with SCD:
61% of ACS cases were not clinically suspected
by physicians prior to radiological diagnosis.
Radiographs should therefore be taken of all patients
with fever, chest pain and respiratory system
symptoms for the detection of ACS.
Elevation in white cell count and decreased hemoglobin
levels have been associated with developing acute
pulmonary complication (4). Similarly in our study,
lower hemoglobin levels were determined in patients
with ACS. Although white cell count was higher
in the ACS group the difference was not significant.
In terms of biochemical tests, Albumin values
were significantly lower in the ACS group. Hypo-albuminemia
is a marker of disease severity and is associated
with poor clinical outcome in acutely ill children.
The decrease in plasma albumin during the acute
phase response is probably due to diminished hepatic
synthesis and the diversion of protein production
required for host defense (14). In the largest
series of its kind, Vichinsky et al. (18) evaluated
671 episodes of ACS in 538 patients to identify
possible etiological factors. They reported no
identifiable cause in 45.7% of cases, while infection
was documented in 29.4% of cases, infarction in
16.1%, and fat embolization in 8.8% (18). We found
no identifiable cause associated with ACS.
All patients with ACS were hospitalized and given
intravenous antibiotics, bronchodilators and analgesia.
The management of ACS is primarily supportive
and includes respiratory therapy, antibiotics,
and, often, erythrocyte transfusion (9,19). Routine,
early transfusions are indicated for patients
at high risk for complications. Those who present
with severe anemia, and multilobar pneumonia should
receive transfusion before respiratory distress
develops. In most patients with anemia, treatment
with leukocyte-depleted, matched, simple transfusions
is safe and effective (18). Transfusion therapy
improves oxygenation within 12 to 24 hours of
erythrocyte transfusion administration. In one
large epidemiological study of ACS, management
with transfusion was associated with a shorter
length of hospitalization. Exchange transfusion
is typically reserved for patients who are not
sufficiently anemic to accommodate a simple transfusion
or those with progressive respiratory decline
or persistent hypoxia despite simple transfusion
(19). In this study, exchange transfusion was
performed on 3 patients due to respiratory difficulty
and hypoxia findings, and a dramatic improvement
was observed. Simple transfusion was performed
on 19 of the 23 patients with ACS. All patients
improved with intravenous antibiotics, bronchodilators,
analgesia and transfusion therapies.
In conclusion, patients with SCD have high basal
CRP and may develop ACS during VOC. Elevated CRP
may herald severe ACS and be significantly related
to risk factors for ACS. Additionally, CRP may
be a good prognostic marker in patients with SCD
and ACS. Overall, these results suggest that further
studies are needed to determine whether CRP can
predict the development of ACS in patients with
VOC.
1. Bakanay SM, Dainer
E, Clair B, Adekile A,
Daitch L, Wells L, Holley
L, Smith D, Kutlar A.
Mortality in sickle cell
patients on hydroxyurea
therapy. Blood. 2005;105(2):545-7.
2. Golden C, Styles L,
Vichinsky E. Acute chest
syndrome and sickle cell
disease. Curr Opin Hematol.
1998;5(2):89-92.
3. Paul RN, Castro OL,
Aggarwal A, Oneal PA.
Acute chest syndrome:
sickle cell disease. Eur
J Haematol. 2011 Sep;87(3):191-207.
Morris C, Vichinsky E,
Styles L. Clinician assessment
for acute chest syndrome
in febrile patients with
sickle cell disease: is
it accurate enough? Ann
Emerg Med. 1999;34(1):64-9.
4. Buchanan ID, Woodward
M, Reed GW. Opioid selection
during sickle cell pain
crisis and its impact
on the development of
acute chest syndrome.
Pediatr Blood Cancer.
2005;45(5):716-24.
5. Akohoue SA, Shankar
S, Milne GL, Morrow J,
Chen KY, Ajayi WU, Buchowski
MS. Energy expenditure,
inflammation, and oxidative
stress in steady-state
adolescents with sickle
cell anemia. Pediatr Res.
2007;61(2):233-8.
6. Mohammed FA, Mahdi
N, Sater MA, Al-Ola K,
Almawi WY. The relation
of C-reactive protein
to vasoocclusive crisis
in children with sickle
cell disease. Blood Cells
Mol Dis. 2010;45(4):293-6.
7. Akinlade KS, Atere
AD, Olaniyi JA, Rahamon
SK, Adewale CO. Serum
copeptin and cortisol
do not accurately predict
sickle cell anaemia vaso-occlusive
crisis as C-reactive protein.
PLoS One. 2013 Nov 4;8(11):e77913.
8. Lionnet F, Hammoudi
N, Stojanovic KS, Avellino
V, Grateau G, Girot R,
Haymann JP. Hemoglobin
sickle cell disease complications:
a clinical study of 179
cases. Haematologica.
2012;97(8):1136-41.
9. Abbas HA, Kahale M,
Hosn MA, Inati A. A review
of acute chest syndrome
in pediatric sickle cell
disease. Pediatr Ann.
2013;42(3):115-20.
10. Castro O, Brambilla
DJ, Thorington B, Reindorf
CA, Scott RB, Gillette
P, Vera JC, Levy PS. The
acute chest syndrome in
sickle cell disease: incidence
and risk factors. The
Cooperative Study of Sickle
Cell Disease. Blood. 1994;84(2):643-9.
11. Bourantas KL, Dalekos
GN, Makis A, Chaidos A,
Tsiara S, Mavridis A.
Acute phase proteins and
interleukins in steady
state sickle cell disease.
Eur J Haematol. 1998;61:49-54.
12. Hedo CC, Aken'ova
YA, Okpala IE, Durojaiye
AO, Salimonu LS. Acute
phase reactants and severity
of homozygous sickle cell
disease. J Intern Med.
1993 ;233(6):467-70.
13. Bargoma EM, Mitsuyoshi
JK, Larkin SK, Styles
LA, Kuypers FA, Test ST.
Serum C-reactive protein
parallels secretory phospholipase
A2 in sickle cell disease
patients with vasoocclusive
crisis or acute chest
syndrome. Blood. 2005;105(8):3384-5.
14. Al-Saqladi AW, Bin-Gadeem
HA, Brabin BJ. Utility
of plasma transferrin
receptor, ferritin and
inflammatory markers in
children with sickle cell
disease. Paediatr Int
Child Health. 2012;32(1):27-34.
15. Vichinsky EP, Haberkern
CM, Neumayr L, Earles
AN, Black D, Koshy M,
Pegelow C, Abboud M, Ohene-Frempong
K, Iyer RV. The Preoperative
Transfusion in Sickle
Cell Disease Study Group.
A comparison of conservative
and aggressive transfusion
regimens in the perioperative
management of sickle cell
disease. N Engl J Med.
1995;333(4):206-13.
16. Bellet PS, Kalinyak
KA, Shukla R, Gelfand
MJ, Rucknagel DL. Incentive
spirometry to prevent
acute pulmonary complications
in sickle cell diseases.
N Engl J Med. 1995;333(11):699-703.
17. Morris C, Vichinsky
E, Styles L. Clinician
assessment for acute chest
syndrome in febrile patients
with sickle cell disease:
is it accurate enough?
Ann Emerg Med. 1999;34:64-9.
18. Vichinsky EP, Neumayr
LD, Earles AN, Williams
R, Lennette ET, Dean D,
Nickerson B, Orringer
E, McKie V, Bellevue R,
Daeschner C, Manci EA.
Causes and outcomes of
the acute chest syndrome
in sickle cell disease.
National Acute Chest Syndrome
Study Group. N Engl J
Med. 2000;342(25):1855-65.
19. Chou ST. Transfusion
therapy for sickle cell
disease: a balancing act.
Hematology Am Soc Hematol
Educ Program. 2013;2013:439-46.
|
|
.................................................................................................................

|
| |
|

