|
|

 |
 |
|
EPIDEMIOLOGY
OF ACUTE RESPIRATORY TRACT INFECTIONS (ARI)
AMONG CHILDREN UNDER FIVE YEARS Old ATTENDING
TIKRIT GENERAL TEACHING HOSPITAL
|
 |
| |
|
Dr Thamer.K.Yousif/MBCh.B/FICMS
DR.BAN A. Khaleq/MSC
|
Acute respiratory tract
infection (ARI) is considered as one of
the major public health problems and it
is recognized as the leading cause of mortality
and morbidity in many developing countries.
The greatest problem for developing countries
is the mortality from ARI in children less
than five year of age (1) .
In most countries, ARI occurs more frequently
than any other acute illness, including
diarrhea and other tropical diseases.
In developing
countries 30% of all patients consultation
and 25% of all pediatric admission are of
ARI ( 3) . Most infections are
limited to the upper respiratory tract and
5% involve the lower respiratory tract.
A large proportion of ARI is present as
pneumonia or bronchiolitis . Incidence of
ARI is almost the same all over the world
: 5-7 episodes per child per years in urban
areas and 3-5 episodes in rural area (
4).
ARI is mostly caused by both viruses and
bacteria. Viral agents account for 90% of
Upper respiratory tract infection (URIs),
however most of these infections do not
result in fatal sever disease; they are
mild and self limited illnesses. While Bacterial
pulmonary infections are common in developing
countries associated with a greater risk
of death (5).
It should be noted that
viral and bacterial infections occur frequently.
Some associated infections include: common
cold, acute otitis media, acute sinusitis,
sore throat, pertussis, bronchiolitis and
pneumonia. (6).
Mortality due to ARI is high in developing
countries which may reach 1000 or more per
100000 live births compared to 30-40 per
100000 live births in industrialized nation
(7). The WHO estimate that in
1990 ARI tragically caused 13 million children
die each year, 4.3 million children die
from ARI, mostly pneumonia, every year in
developing countries. Two- third occurs
in children under one year of age (1),(2).
Billions of children suffer acute or chronic
morbidity arising from their effects. In
all countries ARI is a leading cause of
hospitalization and death. Therefore ARIs
represent a large challenge in field of
communicable diseases (4),(8).
Recognizing the magnitude
of ARI problem, which requires immediate
and concerted action, the WHO has initiated
a global program for its control. The WHO/ARI
program is viewed as critical part of primary
health care and is directed towards children
under the age of five years. Its primary
objective is to reduce the severity and
mortality of pneumonia in children. Other
objectives of the program are to reduce
the incidence of acute lower respiratory
tract infection, to reduce the severity
and complications from acute upper respiratory
infection, and to decrease the inappropriate
use if anti microbial and other drugs for
the treatment of ARI in children (9).
Aim of the study
To study the Epidemiology
of acute respiratory tract infections in
children under five years attending Tikrit
General Teaching Hospital.
Objectives of
the Study
The Study was conducted
to:
- Evaluate the effect
of age, sex and residency on the ARI occurrence
and severity.
- Demonstrate the occurrence
and severity of ARI according to history
of low Birth weight, nutritional status
and immunization status.
- Identify the effect
of feeding pattern on the ARI occurrence
and severity.
- Recognize the relationship
between history of major or chronic illnesses
and ARI occurrence and severity.
- Describe the effect
of maternal and paternal smoking on the
ARI occurrence and severity.
- Assess the relationship
between mother and father educational
level and ARI occurrence and severity.
- Identify the effect
of family history of chronic respiratory
problems and asthma on the ARI occurrence
and severity.
- Estimate the association
between crowding index and the ARI occurrence
and severity.
- Identify the frequency
distribution of clinical signs and symptoms
considered by mothers, and the clinical
signs considered the seriousness of ARI.
- Recognize types
of decision to seek medical advice and
the distribution of ARI cases according
to admission to hospital.
Design
of the Study :
The current work represent a hospital based
longitudinal study, which was conducted
for the period extended from the first of
November 2004 to the end of April 2005 and
with regular working hours.
Socio-Demographic
Characteristics :
The study is conducted in Tikrit General
Teaching Hospital which represents one of
the biggest centers located in the center
of Tikrit city which serves a large proportion
of the community of different socio economic
levels.
Salahaldeen Governorate
has an estimated population of (1162490)
person; Tikrit city represent (159721) of
the population and about (20 %) of those
are children under 5 years of age.
The Study Groups
:
Two thousands four hundreds fifty children
under 5 years of age, who had attended the
hospital for treatment of acute respiratory
tract infections, were collected from the
outpatient clinic.
Pilot Study and
Preset :
A small- scale pilot study was carries out
on a sample of 30 cases including babies
with their parents to identify any areas
of ambiguity in the questionnaire and to
have an idea about time required and other
practical points before final study was
launched.
Another seminar was made in the Community
Medicine Department of the Medical College
of Tikrit University, in the present of
the chairman of the department, the supervisor
of the study, the staff of the department
and post graduate students of the college.
Discussion was made at the end of the seminar,
and comments, notes and suggestions were
given by the audients, which were very wrathful
and helped much in conducting the final
study in more perfect and practical way.
Development of
the Questionnaire and Data Collection:
The questionnaire was developed to collect
the following information:
Age: included three age group (<2) months,
(2-11) months, (12-60) months.
Sex
Type of feeding: which includes type of
feeding for infants lass than 6 months of
age and divided to ; breast feeding only,
bottle feeding only, and bottle and breast
feeding (mixed).
Immunization: the immunization status
of cases was investigated for, BCG, DPT,
polio, Measles, and MMR vaccine and the
children were grouped in to non, partially,
and fully immunization status according
to their age.
History of low birth weight which consider
for infants only.
History of any major or chronic illness.
Residency.
Educational level of parents.
Mother's employment status.
Parental smoking.
Crowding index: number of persons sleeping
in one room (29).
History of chronic respiratory problems
among household.
Family history of asthma.
Type of heating and cooking system.
The knowledge of mothers
of cases regarding the seriousness of ARI
were investigated and their ability to recognize
the following important signs (rapid or
difficult breathing, chest indrawing, nasal
flaring, inability to drink, and becomes
sicker) (48).
(For more detail about questionnaire see
appendix 1)
Examination :
Clinical assessment every case with ARI
was examined for the following points:
- Temperature.
- Respiratory rate:
was determined by inspection of the child
chest for 60 seconds, the RR was counted
twice and the average count was recorded.
- Chest indrawing: by
observing the subcostal and intercostals
space.
- Stridor in calm child.
- Wheeze
Nutritional Status
Examination
The nutritional state of every child was
classified depending on the basis of body
weight for age. So the weight of every child
was measured in kilogram and children with
body weight at or below the 3rd centile
were classified as undernourished child
(133).
Then ARIs cases were
assessed and classified according to WHO/ARI
case management chart into :
A. Very severs disease when child presented
with certain signs such as convulsion, abnormally
sleepy or difficult to wake, stridor in
a calm child, not able to drink, severe
under nutrition.
B. Severe pneumonia when child presented
with chest indrawing with or without fast
breathing.
C. pneumonia when child presented with fast
breathing but no chest indrawing.
D. No pneumonia (cough or cold) no chest
indrawing or fast breathing (7).(
for more detail see appendix 11)
The cases admitted to the wards of the hospital
had been followed up for further information
regarding treatment regime.
Statistical Analysis:
The data collected on (2450) children included
in the study were studied to assess the
association of many risk factors with the
occurrence of ARIs and the association of
many risk factors with the severity of ARIs
cases was also determined.
Conventional statistical techniques were
applied to the data in study of distribution
by frequency percentage and table representation.
The nature of the association studies by
application of statistical tests to measure
the association by help of X2 test with
the value of P less than 0.05 as the limited
level of significance determination of the
difference between percentages.
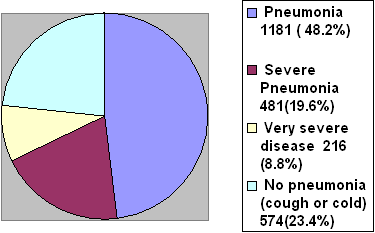
The total sample studied
in this research was (2450) children have
ARI among 5550 children attending to Tikrit
General Teaching Hospital from the first
of November 2004 to end of April 2005 of
the total ARI cases, 216 (8.8%) were classified
as very severe disease, 480 (19.6%) as severe
pneumonia, 1181 (48.2%) as pneumonia, and
573(23.4%) as no pneumonia (cough or cold)
(Figure 1).
The monthly distribution
of cases showed that the lowest frequency
was in April, 147 (6%) followed by March,
221(9%) and Feb., 368(15%) then November,
466(19%) and Jan., 490(20%), while December
showed the highest frequency, 760(31%) (Figure
2).
The study shows that
1539 (62.8%) of all ARIs cases occurred
in the first year of life and ARI was most
frequently diagnosed at the age group (3-13)
months 1279 (52.2%). High significant association
was observed between the age and ARI severity
(Table 1) .
Regarding the gender
the study shows that in 2450 cases of ARIs,
1612 (65.8%) were found to be males and
838 (34.2%) were female and ARIs were 1.5
times higher among males than females. It
showed that no significant association was
observed between ARI severity and gender.(Table
2) .
This study shows that
420 (17.2%) of ARIs cases had history of
LBW. There was statistical significant association
between LBW history and severity of ARIs.(Table
3) .
Regarding Immunization, (Table
.4) shows that 1514 (61.8%) of cases
were fully immunized according to the Immunization
schedule. There was a weak association between
the Immunization status and occurrence of
ARI but a high statistical significance
regarding the severity of ARIs cases (P-value
= 0.0001) .
(Table .4) also
shows that 60% of children who were not
vaccinated at all versus 12.7% of children
who were fully vaccinated had severe type
of ARIs.
Furthermore the study
shows that there was a significant association
between the occurrence of ARIs and undernourished
children. Of all undernourished children,
240 (51.6%) were observed to have severe
diseases (very severe disease and severe
pneumonia). There was a highly significant
association between the undernourished children
and ARI severity.(P-value = 0.0001) (Table.
5) .
The
data on (Table. 6)
demonstrates that, of the total 824 cases
of age less than 6 months, 224 (27.2%) were
breast feeding, 461 (55.3%) were bottle
feeding and 139 (17.5%) were mixed feeding
babies. (Table .6) also shows that breast
feeding provides a highly significant protection
against ARI occurrence and there was a highly
statistical significant association between
ARIs severity and type of feeding (p- value
=0.0001) .
Previous illness history
demonstrates that 417 (17%) of ARI cases
were having history of major or chronic
illness and these children having excessive
risk of about 1.8 times higher than children
without such a history and there was a highly
statistical significant association between
ARI severity and positive history of major
or chronic illness
(Table 7) .
Out of the children with major or chronic
illness, 270 (64.7%) had CHD, 64 (15.3%)
had chronic diarrhea, 49 (11.7%) had Leukemia,
15(3.5%) had TB, 10 (2.4%) had hepatitis
and 10 (2.4%) had chronic renal problem.
Regarding residency distribution
the study shows that most of cases 2078
(84.8%) were from urban areas with no significant
association between area af residency and
the occurrence of ARIs. All cases from rural
areas had pneumonia of different severity
(very severe disease 83 (22.4%), severe
pneumonia 64(17.1%), and pneumonia 225(60.5%)
with strong statistical significant association
between ARIs severity and rural residency
(P-value = 0.0001) (Table
8).
The study shows that,
the maternal smoking had no significant
association with the occurrence of ARI but
have high statistical significant with the
severity of ARI cases. (Table
9).
While the factor of father
smoking was positive in 1332 (54.4%) of
cases with highly significant association
with the occurrence of ARI, but there was
no significance statistical association
observed regarding the severity of ARI cases
with paternal smoking.(Table
10).
The
children of highly educated mothers constituted
only 162(6.6%) of total ARI cases and there
was a significant association between occurrence
of ARI, and mother educational level and
there was a highly significant association
between the mother educational level and
ARI severity (P-value = 0.0001) (Table
11).
While regarding the father educational level
there was also have a highly significant
association with occurrence and severity
of ARI cases. (Table.
12).
This study illustrates
that 363 (14.8%) of the cases have a positive
history of chronic respiratory problems
among household members. Children with such
a history were 4.17 times more likely to
have ARI than the children with a negative
history. But no statistical significance
was observed with severity of ARI cases
(Table 13).
After taking family history
the study shows that, 1275 (52%) of total
ARI cases had family history of asthma.
There was a significant association between
ARI occurrence and family history of asthma.
Also in (Table 14)
it was found that, out of the total children
with positive family history of asthma only
103 (8.1%) were having very severe disease,
while 250 (19.6%), 642 (50.4%), and 280
(21.9%) were having severe pneumonia, pneumonia
and no pneumonia respiratory. There was
no statistical significance association
between illness severity and such history.
(Table 14).
Regarding crowding condition
the study demonstrate that 1000 (40.8%)
cases having a crowding index of (3-5),
but high percentage of case 755 (30.8%)
had crowding index of more than 5. there
was a highly statistical significant relation
between the occurrence and severity of ARI
with living in crowding quarter.
The mothers of the cases
considered the danger signs as follows:
Fever was considered by 2291 (93.5%) mothers
as danger sign, 2276 (92.9%). Mothers agreed
that cough is a danger sign, difficulty
of breathing considered by 449 (44.9%) of
mothers, running nose, wheezing, feeding
difficulty, and rapid breathing were known
by 1017 (41.5%), 953 (38.9%), 877 (35.8%)
and 539 (22%) of mothers respiratory. While
cyanosis, sleep disturbance, drinking difficulty,
convulsion, and chest in drawing, were considered
by small percentage of mothers as danger
signs. (Table. 16)
When the ability of the
mothers to differentiate seriousness of
respiratory disease was investigated, the
study results showed that, 1936 (79%) of
ARIs cases mothers were unable to differentiate
the seriousness of illness by any right
sign or mentioned only one right sign, 461(18.8%)
mothers differentiated 2 right signs and
only 54(2.2%) of the mothers knew 3 right
signs and more.
Many socioeconomic problems
occurred in our country after the last war,
which have different effects on many risk
factors of ARI.
|
|
Regarding
type of heating fuels very high percentage
of families of 2406 cases (98.2%) were depending
on Kerosene in addition to electricity (when
available), while only few families (1%)
of cases were depending on electricity alone.
(0.8%) of cases used wood for heating. Liquid
gas propane was the major sources of fuel
for cooking, 23 families of cases were depending
on wood as source of fuel for cooking. No
further analysis was carried out regarding
types of heating and cooking fuel.
A High percentage of
mothers of cases (98.7%) were housewives.
The study shows that
the dangerous signs in ARI children include
fever (87%), the second is cough that should
be considered as dangerous signs (63%),
while difficulty of breathing was found
in (44.6%) of ARI patients, running nose,
rapid breathing was found in (25.6%) and
(23%) respectively. While wheezing, cyanosis
drinking difficulty, sleep disturbance,
chest in drawing, malnutrition was considered
by small percentage of mothers as a dangerous
sign.(Table 17).
Regarding the hospital
admission the study illustrates that 196
(90.9%), 446 (92.9%), 652(55.2%) , and 176
(30.8%) of patients who had very severe
disease, severe pneumonia, pneumonia , and
no pneumonia respectively were admitted
to hospital during the study period, so
the total patients admitted to hospital
were 1470 (60%), and 980 (40%) treated as
an out patients.(Table
18).
This study shows that
antibiotics were prescribed for 2005 (81.8%)
cases as a part of hospital management,
100% of cases with very severe disease and
severe pneumonia had received antibiotics,
while 985 (83.4%) and 324 (56.4%) of cases
with pneumonia and no pneumonia respectively,
had received antibiotics. Other drugs prescribed
were cough syrup for (61.2%) of patients
and antipyretic for (55%) of patients of
the total cases, 1254 (51.2%) cases received
medications before presenting to hospital,
960 (77.3%) of those children received these
medications by prescriptions from private
doctors, and 172 (13.7%) of cases, by physicians
in health centers, and 112 (8.9%) of cases
received these medications without any medical
prescription. Of these 1254 children, 157
(12.5%) cases received cold medications
only, 221 (17.6%) cases received antibiotics
only, and 877 (69.9%) cases received both
antibiotics and cold medications.
So, 1098 (44.8%) of total
ARI cases received antibiotics before presenting
to hospital 161 (14.7%) of them had very
severe disease, 231 (21%) had severe pneumonia,
519 (47.3%) had pneumonia , and 187 (17%)
had no pneumonia ( cough or cold) at time
of examination.
when the child gets an ARI attack, the mother
would take him to an private clinic in (42.8%)
of cases , to health center in (14.4%),
hospital in (12.0%), public clinic in (12.0%),
only home management in (10.6%) using drugs
from pharmacy in (6%), and would do nothing
in (2.2%) only.
This means that (81.2%) of mothers will
seek medical advice.(Table
20).
Acute
respiratory tract infection (ARI) are one
of the most commonest causes of health problems
with high morbidity and mortality among
children under five years old particularly
in developing countries (147).
Moreover, these infections place a heavy
burden on the health services in terms of
utilization of hospitals and health centers
(17),(10).
In Iraq ARI control programme had been adopted
since 1990 (9). For success of
such a programme, ARI epidemiology is needed
to be known which help to improve methods
of management of these illnesses (148).
The highest frequency of ARIs cases were
observed to be found in December. This is
comparable to the results found by International
consultation on the control of ARI in 1991(61)
. It might be due to overcrowding rather
than climate (61), or due to
seasonality of infective agents themselves
(116).
ARIs were more frequent among infants in
the present study. Early infancy less than
2 months of age has a highly significant
association with severe ARI. These findings
were in agreement with the result found
by Qasim, AL-Jassar, AL-Karaguily, AL-Humairy,
AL-Azzawi in Iraq (53),(32),(54),(55),(56).
This might be due to the fact that immunity
has not become fully established, narrow
airways, incomplete development of the lungs
and relatively short bronchial tree (50),(124)
.
Male patients were more infected with ARI
than female. They were 1.5 times more likely
to develop ARIs than female patients, but
there was no significant association between
gender and the severity of ARIs. These findings
were consistent with AL-Jassar and AL-Karaguily
in Iraq (32),(54), but against
the result found by Zhang et al in Beijing
and Ali in Iraq (63),(64), and
also against the result found by AL-Humairy
in Iraq (55) who found that male
gender was highly significant associated
with ARI severity.
Infants with a history of low birth weight
appeared to has significant association
with ARIs occurrence and severity. This
was in agreement with Taylor, et al, chain
et al in Japan and AL-Jassar in Iraq (75),(32),(76),
this might be due to low birth weight baby
has a poor pulmonary function and low immunity,
which makes them more liable to have ARI
mainly in its severe picture (131).
This finding was against that found by Saddam
and Al-Tawil in Iraq (62), in
which low birth weight was not observed
to be as a significant factors for ARI severity
.
Immunization showed weakly association with
the occurrence of ARI. This could be due
to fact that there was lack of vaccination
either partial or complete of the cases
and this might be due to the events that
occurred after the last war and invasion
on our country. But immunization appears
to be strongly associated with severity
of ARI cases. This result was in agreement
with results found by Broor et al in India
and Al-Humairy in Iraq (87),(55).
The results showed that there are significant
association regarding the nutritional status
on occurrence and severity of ARI. This
observation was documented by AL-Humairy
in Iraq, Rahman & Rahman in Bangladesh,
Tupasi and Hamid in Southeast Asia (55),(80),(78),(79).
This could be explained by that the immunologic
insufficiency which is common in malnutrition
lead to infection (13),(50).
Significant
association had been observed regarding
type of feeding and the development of ARI,
that is breast feeding appeared to be highly
protective against the occurrence of ARI.
This finding was also noted in Perera, Nilay
et al in Turkey, Shah et al in South Kerala
and Fonseca in Fortaleza in Brazil. But
against finding of AL-Jassar in Iraq who
found that breast feeding was not significantly
protective against the development of ARI,
(67),(68), (70),(147).
The cause of protection of human milk is
due to its content of bacterial and viral
antibodies, macrophages synthesizing complement
and lysozymes (35). There were
a significant role of different types of
feeding appeared to play on the severity
of ARI cases, this result was agreed the
result found by AL-Jassar in Iraq (32),
who found that breast feeding prevent severe
forms of ARI . Illness severity might be
aggravated by several factors like poor
hygiene, home environment and maternal care
(130).
Strong association had been observed regarding
positive child history of chronic or major
illness and the occurrence and severity
of ARI in this present study. Children with
such history had (1.84) higher rate of ARIs
than those children with no such a history.
This observation was documented by AL-Jassar
and Qasim in Iraq and Mc Millan et al in
USA (32),(53),(52). This could
be explained by that, children with chronic
or major illness have a general ill health,
impaired immunity and poor nutrition (124).
Heart disease is associated with excessive
pulmonary blood flow, which is responsible
for recurrent pulmonary infection in addition
to poor general nutrition and impaired host
defense, so children with congenital heart
disease were more prone to severe ARIs (62).
Considering area of residence whether rural
or urban, there was no significant association
between ARIs and residency. This was in
agreement with AL-Shahabi et al in Iraq
(57), but against the result
found by Pio et al in America and UNICEF\WHO
statement program for the control of ARI
in developing countries in 1986 (130),(34).
Regarding the severity of ARI cases in this
study, rural areas appeared to play an important
role, in which there was a highly significant
association between ARIs severity and rural
residency. This may be explained by the
fact that cases of severe ARIs residing
in rural areas are usually referred to hospital
for admission, while less severe cases are
usually treated in the primary areas. This
result was documented with WHO\ARI program
of control of acute respiratory infections
in 1991 and Saddam & AL_Tawil in Iraq
(104),(62).
The
present study demonstrated that there was
no association observed with ARI development
of ARI cases and maternal smoking, but have
a high association with ARI severity.
This result was agreed with Nillay et al
in Turkey and Qasim & AL-Jassar in Iraq.(
68),(53),(32) but against the result
found by ARI News letter in Egypt, Fergusson
et al and Lopez et al in USA (24),(110),(108).
This might be due to the fact that smoking
habit among female is not much accepted
in our society, this leads to small number
of smoking mothers, therefore the real association
can not be demonstrated in the present study,
but if a mother is smoker it will lead to
aggravate the condition of the ARI to severe
form.
Paternal smoking found to be highly associated
with occurrence of ARI, this finding was
against the result found by Fergusson et
al in USA (108) that maternal
smoking was strongly correlated with children
respiratory diseases than paternal smoking,
but our results of association of paternal
smoking and occurrence of ARI was in agreement
with result found by Rahman and Rahman in
Bangladesh and Biswas et al in India (80),(107).
Passive smoking can act by increasing the
rate of cross infection from the smokers,
mediating an allergic reaction, or by causing
irritation of the infantile passages and
facilitate the spread of infection to lower
respiratory tract (98). There was significant
association observed between the paternal
and smoking and ARI severity. This was found
by Al-Humairy in Iraq (55).
There was a strong association between the
parent's education and the occurrence of
ARI in the present study. This finding agreed
with Lopez et al, Hamid et al, Nillay in
Turkey (110),(79),(68). The protective
effect of parental education against acute
respiratory infection awareness and care
practices. This result was in disagreement
with AL-Jassar in Iraq (32) who
found that father's education play no role
regarding the severity of ARI.
This present study demonstrated a highly
significant association of chronic respiratory
problems among household members and ARIs
and it was found that , children with such
a family history were 4.17 times more risky
to develop ARI than children without that
history. This result agreed with Pedrerta
et al, Ware et al in America & Broor
et al in India (115),(114),(87),
but against the result reported by Qasim
and AL-Jassar in Iraq (53),(32),which
suggest that family history of chronic respiratory
problems plays no role either in the occurrence
or in the severity of ARI,. Also this study
found that such a history plays no role
in the severity of ARI, in which the study
result showed that more than two-thirds
of cases had mild forms of ARI ( no pneumonia
and pneumonia).
Strong
association appeared between children from
families with a positive history of asthma
and ARI occurrence. This was documented
by Lopez et al and Mc Connchie et al in
America (110),(92). But the present
study demonstrated that there was no significant
association regarding the severity of ARI.
This result agreed with AL-Jassar in Iraq
(32).
The present study showed that children living
in overcrowded houses were more liable to
ARI than those children living in less crowded
houses. This result coincided with the results
in Rahman and Rahman in Bangladesh and Azize
et al in Malaysia (80),(69),
but against the result found by Nilay et
al in India (68), Study in which there was
no association between the number of persons
per room and ARI occurrence. The results
of this study also found that overcrowded
houses had a highly significant association
with development of severe ARI. This result
was also demonstrated by AL-Shahabi et al
in Iraq (57).
A high percentage of mothers identifying
the seriousness of ARI that paid their attention
for seeking medical advice were fever and
cough .
While fast breathing, chest indrawing, convulsion,
drinking difficulties cyanosis, and sleep
disturbance recognized by a small percentage
of mothers. This result was against the
result found by Rashid et al in Bangladesh
(148) study in which most of
mothers recognized pneumonia and all mild
and severe signs and symptoms of pneumonia.
This result was also against the result
found by Mohammed in Iraq (125),
but in agreement with results of WHO\ARI
program of control of ARI in 1991, AL-Tae'e
in Iraq, Gve and Aung et al in Southern
Asian when mothers were not able to recognize
serious ARI signs and symptoms from the
simple one (104),(145),(141),(149).
This could be due a defect in health education
aspect regarding ARI control program and
also could be due to low educational level
of mothers in the study sample in which
about more than half of mothers were either
illiterate or only have primary level of
education.
The present study found that about half
of ARI cases had received a medications
before presenting to hospital and (91.1%)
of those children used these medications
by prescription from doctors and only (8.9%)
of them were using medications without any
medical prescription.
This finding can reflect that mothers were
more relying upon medical advises, and this
should be further encouraged by education
about the harm of misusage drugs without
medical prescription.
Regarding antibiotic, of all medications
antibiotics were used in (44.8%) of cases.
Almost physicians prescribe antibiotics
to reduce the number of return office of
bacterial infections (138). The
prescription of antibiotics for ARI cases
were not preventing them from reaching the
hospital, this could be due to misusage
of these antibiotics for viral infections
or misusage by parents. This result is in
agreement with result found by Pichichero
et al in USA (138).
Antibiotics were prescribed unnecessarily
for ARI patient. In present study, antibiotics
had been prescribed for (81.8%) of ARI cases
as a part of hospital management, by application
of WHO- ARI case management chart ( Appendix
11). This rate can be reduced to 76% were
prescribed unnecessarily for 5.2% of ARI
cases which is lower than that found by
AL-Jassar in Iraq & Gunha et al in Rio
de Janeiro (32),(137), which
were 12.4% and 8.9% respectively.
This may reflect the increases in the awareness
among medical staff regarding the unnecessary
and harmful use of antibiotics and the ARI
control program adopted by the ministry
of health.
Antipyretic and cough syrup were other drugs
used for the treatment of ARIs cases as
a part of hospital management. These two
drugs might be useful to relieve symptoms,
although most children with cold or cough
need no drugs at all (122).
Many cases admitted to hospital can be treated
as an outpatients. The admission rate of
ARI cases was 60% this rate can be reduced
to 28.4% when WHO case management criteria
for ARI cases were applied because 56.3%
of cases admitted to hospital can be treated
as an outpatients. This reduction is very
important because it will decrease the burden
imposed upon hospital (126).
The result obtained from the study showed
that a high percentage of mothers identifying
the seriousness of ARI that paid their attention
for seeking medical advice were fever and
cough, while chest indrawing, convulsion,
drinking difficulties Sleep disturbance
was recognized by small percentage of mothers.
This is in agreement with Gue and Aung in
Southern Asian, Al-Jassar in Iraq and WHO\ARI
program of control of ARI in 1991 where
mothers were not able to recognize serious
ARI signs from the simple ones (141),(104),(32),(149).
This may indicate that Iraqi mothers are
not different from mothers else where who
had Considered fever and cough as an important
signs that needed medical attention, and
this might be due to the defect in health
education aspect of ARI control program
adopted by the ministry of health regarding
dangerous sign (150).
Pneumonia
was the most common form of ARI observed
among the study cases, and the higher frequency
of ARI was observed during December.
Infancy, history of LBW in infants, under
nutrition, lack of immunization, absence
of breast-feeding in the first six months
of life, educational level of parents, child
history of major or chronic disease, living
in crowded quarter & Paternal smoking
were observed to be important risk factors
for both, development and severity of ART
Male gender and family history of chronic
respiratory problems were found to be responsible
for the occurrence of ARI but play no significant
role in its severity.
Rural
residences were found to have a significant
effect on ARI severity. In this study the
effect of maternal smoking on the development
and severity of ARI was not clearly demonstrated.
Fever,
cough and breathing difficulty were considered
by mothers as an important signs for ARI
which makes them look for medical advice.
There is still a great lack of knowledge
about ARI program among public, that is
most of mothers were not able to recognize
the dangerous signs of ARI.
Antipyretics
and antibiotics were the most common types
of medications used by cases before presenting
to hospital, but this practice did not prevent
the children from reaching the hospital.
This may indicate a misusage of these antibiotics
either by doctors or by families.
There
was decreased in the prescription of unnecessarily
antibiotics by hospital physicians.
The
goal of ARI programme still not fully achieved
and there is a real lack of effective ARI
education programmes, as the majority of
mothers were unable to recognize the dangerous
signs of ARI. However, more information
messages are needed to increase mothers
ability to differentiate between mild and
sever cases.
Mass media especially radio and TV, play
an important role in distributing a scientific
information among the population. So attention
should be paid for the quality of information
given to mothers through mass media .
Recommendations
Strengthening of ARI programme in order
to make its message reaches the majority
of Iraqi mothers through the following :
|
a.
|
Mass
media
|
| b. |
Training
of doctors and medical workers |
| c. |
Integration
of the programme with Non Governmental
Organization (NGOS). |
| d. |
The
ARI programme must share the health
educational department in Ministry of
Health during planning Implementation
and evaluation for continuous medical
education program which enable the medical
staff to operate their scientific and
medical information. |
| e. |
To
have an ARI unit PHC center, specially
in low standard of living areas high
density population and low level of
education. |
| 6.2.2.
: |
Improvements
in the case management skills of locally
adapted guidelines provision management
of childhood illness and activities
to promote through the health staff
on integrated their use. |
| 6.2.3
: |
Pediatricians
and general practitioners should take
their role in the process of education
of families and particularly the mothers
about the following :
a. Causes of acute respiratory illness
b. Risk factors of ARI
c. Dangerous signs of ARI illness and
the importance of simple supportive
therapy as well as the early referral
of child with severe illness.
d. The value of timely and complete
immunization and the importance of breast-feeding.
e. Avoidance of taking medications without
medical prescription
This practice should be as a part of
management process even at the private
clinics .
|
|
|
Figure 1: Distribution of Cases According
to ARI Severity at Examination.
Figure
2:
The Monthly Distribution of Cases ARI Presented
to
Hospital From the First of November 2004,
to the End of April 2005.
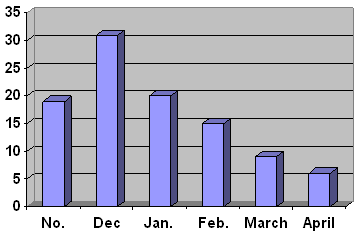
<back
to text
Table
1: Age Distribution of ARI Cases and
Distribution of Cases According to Age and
ARI Severity.
|
Age
|
Case
|
Severity of ARI
|
| Very
Severe Disease |
Severe
Pneumonia |
Pneumonia |
No
Pneumonia |
| |
No |
% |
No |
% |
No |
% |
No |
% |
No |
% |
| <2m |
260 |
10.6 |
54 |
20.8 |
24 |
9.4 |
172 |
66 |
10 |
3.8 |
| 2-11m |
1279 |
52.2 |
102 |
8 |
283 |
22.2 |
663 |
51.8 |
230 |
18 |
| 12-60m |
911 |
37.2 |
60 |
6.5 |
173 |
19 |
346 |
38 |
333 |
36.5 |
| Total |
2450 |
100 |
216 |
8.8 |
480 |
19.6 |
1181 |
48.2 |
573 |
23.4 |
Chi-sq = 233.405
DF = 6
P-value = 0.0001
<back
to text
Table
2: Gender Distribution of ARI Cases
and Distribution of Cases According to Sex
and ARI Severity.
|
Sex
|
Case
|
Severity of ARI
|
| Very
Severe Disease |
Severe
Pneumonia |
Pneumonia |
No
Pneumonia |
| |
No |
% |
% |
% |
No |
% |
No |
% |
No |
% |
| male |
1612 |
65.8 |
147 |
9.1 |
319 |
19.8 |
788 |
48.9 |
358 |
22.2 |
| female |
838 |
34.2 |
69 |
8.2 |
161 |
19.3 |
393 |
46.8 |
215 |
25.7 |
| Total |
2450 |
100 |
216 |
8.8 |
480 |
19.6 |
1181 |
48.2 |
573 |
23.4 |
Chi-sq = 3.837
DF = 3
P-value = 0.280
<back
to text
Table
3 : Distribution of ARI Cases According
to History of LBW and Distribution of Cases
According to History of LBW and Illness
Severity.
|
History of Low
birth Weight
|
Case
|
Severity of ARI
|
| Very
Severe Disease |
Severe
Pneumonia |
Pneumonia |
No
Pneumonia |
| |
No |
% |
% |
% |
No |
% |
No |
% |
No |
% |
| LBW |
420 |
17.2 |
54 |
12.9 |
162 |
38.9 |
109 |
25.9 |
94 |
22.3 |
| Normal |
2030 |
82.8 |
250 |
12.3 |
422 |
20.8 |
788 |
38.8 |
570 |
28.1 |
| Total |
2450 |
100 |
304 |
12.4 |
585 |
23.9 |
897 |
36.6 |
664 |
27.1 |
Chi-sq = 67.316
DF =3
P-value = 0.0001
<back
to text
Table
4: Distribution of ARI Cases According
to the Immunization Status and Distribution
of Cases According to Immunization Status
and Illness Severity.
|
Immunizations
Status
|
Case
|
Severity of ARI
|
| Very
Severe Disease |
Severe
Pneumonia |
Pneumonia |
No
Pneumonia |
| |
No |
% |
No |
% |
No |
% |
No |
% |
No |
% |
| Fully |
1514 |
61.8 |
55 |
3.6 |
138 |
9.1 |
901 |
59.5 |
421 |
27.8 |
| Partially |
617 |
25.2
|
73 |
11.9 |
240 |
38.9 |
181 |
29.4 |
122 |
19.8 |
| Not |
319 |
13 |
88 |
27.7 |
102 |
32.3 |
99 |
30.8 |
30 |
9.2 |
| Total |
2450 |
100 |
216 |
8.8 |
480 |
19.6 |
1181 |
48.2 |
573 |
23.4 |
Chi-sq = 555.705
DF= 6
P-value = 0.0001
<back
to text
Table
5: Nutritional Status of ARI Cases According
to Distribution of Cases According to Nutritional
Status and ARI Severity.
|
Nutrition Status
|
Case
|
Severity of ARI
|
| Very
Severe Disease |
Severe
Pneumonia |
Pneumonia |
No
Pneumonia |
| |
No |
% |
No |
% |
No |
% |
No |
% |
No |
% |
| Under-
nourished |
464 |
19 |
108 |
23.2 |
132 |
28.4 |
147 |
31.6 |
77 |
16.8 |
| Normal |
1986 |
81.0 |
108 |
5.4 |
348 |
17.5 |
1034 |
52.1 |
496 |
25.0 |
| Total |
2450 |
100 |
216 |
8.8 |
480 |
19.6 |
1181 |
48.2 |
573 |
23.4 |
Chi-sq = 200.664
DF = 3
P-value = 0.0001
<back
to text
Table
6: Feeding Pattern of ARI Cases and
Distribution of Cases According to Feeding
Pattern and Illness Severity.
|
Type of Feeding
|
Case
|
Severity of ARI
|
| Very
Severe Disease |
Severe
Pneumonia |
Pneumonia |
No
Pneumonia |
| |
No |
% |
No |
% |
No |
% |
No |
% |
No |
% |
| Bottle
only |
461 |
55.3 |
89 |
18.4
|
108 |
23.7 |
140 |
30.7 |
124 |
27.2 |
| Breast
only |
224 |
27.2 |
20 |
8.9 |
36 |
16.1 |
80 |
35.7 |
88 |
39.3 |
| Mixed |
139 |
17.5 |
31 |
25.0 |
102 |
32.3 |
99 |
30.8 |
30 |
9.2 |
| Total |
824 |
100 |
140 |
17.0 |
48 |
33.3 |
40 |
27.8 |
20 |
13.9 |
Chi-sq = 46.130
DF= 6
P-value = 0.0001
<back
to text
Table
7: History of Major or Chronic Illnesses
of Cases and Distribution of Cases According
to Such History and ARI Severity.
|
Child history
of major or chronic illness
|
Case
|
Severity of ARI
|
| Very
Severe Disease |
Severe
Pneumonia |
Pneumonia |
No
Pneumonia |
| |
No |
% |
No |
% |
No |
% |
No |
% |
No |
% |
| Yes |
417 |
17.0 |
98 |
23.5 |
78 |
18.9 |
182 |
43.5 |
59 |
14.1 |
| No |
2033 |
83.0 |
118 |
5.8 |
402 |
19.8 |
999 |
49.1 |
514 |
25.3 |
| Total |
2450 |
100 |
216 |
8.8 |
480 |
19.6 |
1181 |
48.2 |
573 |
23.4 |
Chi-sq
= 143.545
DF = 3
P-value = 0.0001
<back
to text
Table
8: Residency Distribution of Cases and
Distribution of Cases According to Residency
Distribution and ARI Severity.
|
Residency
|
Case
|
Severity of ARI
|
| Very
Severe Disease |
Severe
Pneumonia |
Pneumonia |
No
Pneumonia |
| |
No |
% |
No |
% |
No |
% |
No |
% |
No |
% |
| Urban |
2078 |
84.8 |
133 |
6.4 |
416 |
20.0 |
956 |
46.0 |
573 |
27.6 |
| Rural |
372 |
15.2 |
83 |
22.4 |
64 |
17.1 |
225 |
60.5 |
0 |
0 |
| Total |
2450 |
100 |
216 |
8.8 |
480 |
19.6 |
1181 |
48.2 |
573 |
23.4 |
Chi-sq
= 208.179
DF= 3
P-value = 0.0001
<back
to text
Table
9: Distribution of ARI Cases According
to Maternal Smoking and Distribution of
Cases According to Maternal Smoking and
ARI Severity.
|
Mother
Smoking
|
Case
|
Severity of ARI
|
| Very
Severe Disease |
Severe
Pneumonia |
Pneumonia |
No
Pneumonia |
| |
No |
% |
No |
% |
No |
% |
No |
% |
No |
% |
| Yes |
99 |
4.0 |
16 |
15.0 |
10 |
10.0 |
64 |
65.6 |
9
|
10.0 |
| No |
2351 |
96.0 |
200 |
8.5 |
470 |
20.0 |
1117 |
47.5 |
564 |
24.0 |
| Total |
2450 |
100 |
216 |
8.8 |
480 |
19.6 |
1181 |
48.2 |
573 |
23.4 |
Chi-sq = 25.860
DF = 3
P-value = 0.0001
<back
to text
Table
10: Distribution of ARI According to
Paternal Smoking and Distribution of Cases
According to Paternal Smoking and ARI Severity.
|
Father
Smoking
|
Case
|
Severity of ARI
|
| Very
Severe Disease |
Severe
Pneumonia |
Pneumonia |
No
Pneumonia |
| |
No |
% |
No |
% |
No |
% |
No |
% |
No |
% |
| Yes |
1332 |
54.4
|
109 |
8.1 |
234 |
17.6 |
646 |
48.5 |
343 |
25.8 |
| No |
1118 |
45.6 |
107 |
9.6 |
246 |
22.0 |
535 |
47.8 |
230 |
20.6 |
| Total |
2450 |
100 |
216 |
8.8 |
480 |
19.6 |
1181 |
48.2 |
573 |
23.4 |
Chi-sq = 14.454
DF = 3
P-value = 0.002
<back
to text
Table
11: Mother Educational Level of Cases
and Distribution of Cases According to Mother
Educational Level and ARI Severity.
|
Educational
Level of Mother
|
Case
|
Severity of ARI Cases
|
|
Very
Severe Disease
|
Severe
Pneumonia
|
Pneumonia
|
No
Pneumonia
|
|
No
|
%
|
No
|
%
|
No
|
%
|
No
|
%
|
No
|
%
|
|
Illiterate
|
623
|
25.4
|
74
|
12.0
|
103
|
16.5
|
387
|
62.1
|
59
|
9.4
|
|
Primary
|
891
|
36.4
|
98
|
11.0
|
196
|
22.0
|
392
|
44.0
|
205
|
23.0
|
|
Secondary
|
774
|
31.6
|
44
|
5.7
|
157
|
20.3
|
343
|
44.3
|
230
|
29.7
|
|
Higher
|
162
|
6.6
|
0
|
0
|
24
|
15.1
|
59
|
36.4
|
79
|
48.5
|
|
Total
|
2450
|
100
|
216
|
8.8
|
480
|
19.6
|
1181
|
48.2
|
573
|
23.4
|
Chi-sq
= 186.921
DF
= 9
P-value = 0.0001
<back
to text
Table
12: Father Educational Level of Cases
and Distribution of Cases According to Father
Educational Level and ARI Severity.
|
Educational
Level of Father
|
Case
|
Severity of ARI Cases
|
|
Very
Severe Disease
|
Severe
Pneumonia
|
Pneumonia
|
No
Pneumonia
|
|
No
|
%
|
No
|
%
|
No
|
%
|
No
|
%
|
No
|
%
|
|
Illiterate
|
397
|
16.2
|
54
|
13.6
|
88
|
22.2
|
226
|
56.8
|
29
|
7.4
|
|
Primary
|
725
|
29.6
|
73
|
10.1
|
196
|
27.0
|
363
|
50.0
|
93
|
12.9
|
|
Secondary
|
911
|
37.2
|
64
|
7.0
|
147
|
16.1
|
411
|
45.1
|
289
|
31.8
|
|
Higher
|
417
|
17.0
|
25
|
5.8
|
49
|
11.7
|
181
|
43.5
|
162
|
39.0
|
|
Total
|
2450
|
100
|
216
|
8.8
|
480
|
19.6
|
1181
|
48.2
|
573
|
23.4
|
Chi-sq = 217.912
DF = 9
P-value = 0.0001
<back
to text
Table
13: Distribution of ARI Cases According
to Family History of Chronic Respiratory
Problems and Distribution of Cases According
to Such History with ARI Severity.
|
Chronic
Respiratory Problems Among Household
Members
|
Case
|
Severity
of ARI Cases
|
|
Very
Severe Disease |
Severe
Pneumonia |
Pneumonia |
No
Pneumonia |
|
No |
% |
No |
% |
No |
% |
No |
% |
No |
% |
|
Yes |
363 |
14.8 |
39 |
10.8 |
79 |
21.6 |
138 |
37.9 |
107 |
29.7 |
|
No |
2087 |
85.2 |
177 |
8.5 |
401 |
19.2 |
1043 |
50.0 |
466 |
22.3 |
|
Total |
2450 |
100 |
216 |
8.8 |
480 |
19.6 |
1181 |
48.2 |
573 |
23.4 |
Chi-sq
= 18.751
DF = 3
P-value
= 0.05
<back
to text
Table
14: Distribution of ARI Cases According
to Family History of Asthma Distribution
of Cases According Such History with ARI
Severity
|
Family
History of Asthma
|
Case
|
Severity of ARI Cases
|
|
Very
Severe Disease |
Severe
Pneumonia |
Pneumonia |
|
No |
% |
No |
% |
No |
% |
No |
% |
|
Yes
|
1275 |
52.0 |
103 |
8.1 |
250 |
19.6 |
642 |
50.4 |
|
No |
1175 |
48.0 |
113 |
9.6 |
230 |
19.6 |
539 |
45.8 |
| Total |
2450 |
100 |
216 |
8.8 |
480 |
19.6 |
1181 |
48.2 |
Chi-sq = 6.504
DF = 3
P-value = 0.090
<back
to text
Table
15: Distribution of ARI Cases According
to Crowding Index and Distribution of Cases
According to Crowding Index and ARI Severity
|
Crowding
Index |
Case
|
Severity
of ARI Cases
|
|
Very
Severe Disease |
Severe
Pneumonia |
Pneumonia |
No
Pneumonia |
|
No |
% |
No |
% |
No |
% |
No |
% |
No |
% |
|
<3 |
695 |
28.4 |
34 |
4.9 |
132 |
19.1 |
392 |
56.3 |
137 |
19.7 |
|
3~5 |
1000 |
40.8 |
74 |
7.4 |
206 |
20.6 |
416 |
41.6 |
304 |
30.4 |
|
>5 |
755 |
30.8 |
108 |
14.3 |
142 |
18.8 |
373 |
49.4 |
132 |
17.5 |
|
Total |
2450 |
100 |
216 |
8.8 |
480 |
19.6 |
1181 |
48.2 |
575 |
23.4 |
Chi-sq
= 96.351
DF
= 6
P-value
=- 0.0001
<back
to text
Table
16: Frequency Distribution of Clinical
Signs and Symptoms Considered by Mothers
to Reflect the Seriousness of ARI
|
Signs
and Symptoms |
Number
of Mothers |
% |
|
Fever
Cough
Breathing difficulty
Running nose
Wheezing
Feeding difficulty
Rapid breath
Cyanosis
Sleep disturbance
Drinking difficulty
Conclusion
Chest in drawing
|
2291
2276
1100
1017
953
877
539
211
100
96
10
5
|
93.5
92.9
44.9
41.5
38.9
35.8
22
8.6
4.1
3.9
0.4
0.2
|
<back
to text
Table
17: The Clinical Signs Considered the
Seriousness of ARI
|
Dangerous
sign |
No |
% |
|
Fever
Cough
Breathing difficulty
Running nose
Rapid breath
Feeding difficulty
Wheezing
Bluish
Drinking difficulty
Sleep disturbance
Chest in drawing
Malnutrition
|
2132
1553
1093
627
564
279
162
123
74
74
25
20
|
87%
63.4%
44.6%
25.6%
23.0%
11.4%
6.6%
5%
3%
3%
1%
0.8%
|
<back
to text
Table
18: Distribution of ARI Cases According
to Admission to Hospital and Disease Severity
|
Hospital Admission
|
Very Severe Disease
|
%
|
Severe Pneumonia |
% |
Pneumonia |
% |
No. Pneumonia |
% |
Total |
| Yes |
196 |
90.9 |
446 |
92.9 |
652 |
55.2 |
176 |
30.8 |
1470 |
| No |
20 |
9.1 |
34 |
7.1 |
529 |
44.8 |
397 |
69.2 |
980 |
| Total |
216 |
100 |
480 |
100 |
1181 |
100 |
573 |
100 |
2450 |
Chi-sq =517.800
DF = 3
P-value = 0.0001
<back
to text
Table
19: Distribution of Antibiotic Uses
in ARI Cases According to Illness Severity
|
Antibiotic
Uses |
Severity
of ARI Cases |
|
Very
Severe Disease |
% |
Severe
Pneumonia |
% |
Pneumonia |
% |
No
Pneumonia |
% |
Total |
| Yes |
216 |
100 |
480 |
100 |
985 |
83.4 |
324 |
56.4 |
2005 |
| No |
0 |
0 |
0 |
0 |
196 |
16.6 |
249 |
43.6 |
445 |
| Total |
216 |
100 |
480 |
100 |
1181 |
100 |
573 |
100 |
2450 |
Chi-sq = 403.022
Df = 3
P- value = 0.0001
<back
to text
Table
20: Type of Decision to Seek Medical
Advice
|
Treated by |
No |
% |
|
Private clinic |
1049 |
42.8% |
|
Health center |
356 |
14.4% |
|
Hospital |
294 |
12.0% |
|
Public clinic |
294 |
12.0% |
|
Home management |
260 |
10.6% |
|
Do nothing |
54 |
2.2% |
|
Drug from pharmacy |
147 |
6% |
<back
to text
|
|
|
|
| 1. |
Bashour
HN, Roger HW, Thomas F. a community
- based study of acute respiratory infection
among preschool children in Syria. Journal
of tropical pediatrics. 1994 ; 40:207-211. |
| 2. |
WHO\CDR.
Out patient management of young children
with ARI. Epidemiology and etiology
of acute respiratory infections. (1995.b). |
| 3. |
Anonymous:
Epidemiology of acute respiratory tract
infections. Bulletin of the world Health
organization. 1998; 76: 101-103. |
| 4. |
WHO
Viral respiratory diseases. WHO technical
respiratory series. 1980; 64:21-63. |
| 5. |
Importance
of respiratory viruses in acute otitis
media. April 2003. Retrieved 1 December,
2004, from http: //www. Google. Internet
/ / Tips for better browsing / CMR.
Clinical microbiology reviews. |
| 6. |
Hyam
N. Bashour, Roger H. Webber, and Thomas
F. A community- based study of acute
respiratory infection among pre school
children in Syria. Journal of tropical
pediatrics. 1994 ; 40:207-211. |
| 7. |
WHO\
ARI. Acute respiratory infections in
children : facts and figures on acute
respiratory infections in children.
(1990.a). |
| 8. |
Douglas
R M. the Cinderella of communicable
diseases, in acute respiratory infections
in childhood. Sydney, International
Workshop publication of university of
Adelaide ; 1985: 201-209. |
| 9. |
WHO/
ARI. Program of control of acute respiratory
infections. Interim program report.
(1991.b). |
| 10. |
WHO.
Training course. CDD\ ARI program management
. WHO division of diarrheal and ARI
control (1995.a). |
| 11. |
Whaley
L, Wong D. Essential of pediatric nursing.
International Journal of Epidemiology.
1995; 2: 609-611. |
| 12. |
WHO/
ARI. Management of the young child with
acute respiratory infections. Supervising
skills. (1991.a). |
| 13. |
Beherman
R E, Klieg man R M, Arivan A M. infections
disease. In: Behreman R E, Kligman J,
Jenson M. Nelson Text book of pediatric.
17 ed. China, international publication;
2000: 1236-1250. |
| 14. |
Lye
M S, Iai K P, Kaur H, et al. Acute respiratory
infections in Malaysian children. Journal
of tropical pediatrics. 1994 ; 40:1-30. |
| 15. |
Michel
Gavenne, Caroline Ronsmans and Harry
Campbell. The magnitude of mortality
from acute respiratory infections in
children under 5 years in developing
countries. World health statistics.
1992; 45: 33-45. |
| 16. |
WHO\
CDR. Introduction- outpatient management
of young children with ARI. (1995.c). |
| 17. |
WHO\
report on ARI (1995). |
| 18. |
Keena
M. The ear. In Beherman E, Kligman M,
Tonson B, eds. Nelson Textbook of pediatric.
16th ed. China, W B Saunders company
; 2000: 1939-1962. |
| 19. |
Todd
J K. Infections disease. In: Behrman
Richard E, Kliegman Robert M, Jenson
Hal B, eds. Nelson textbook of pediatrics.
16th ed. China, W B Saunders Company;
2000:376-1100. |
| 20 |
Principi
N, Marchisio P, Tornaghi R, Massironi
E, Sonority J, Picco P, et al . Occurrence
of infections in children infected with
human immune deficiency virus. Pediatric
infections disease Journal . 1991 ;
10:190-193. |
| 21. |
Stansfield
S K, Shepard D S. acute respiratory
infections in: Jamison D T, Mosley H
W, Measham A R, eds. Disease control
priorities in developing countries.
New York, Oxford university press publication:
1993:67-90. |
| 22. |
Klugman
K P. childhood HIV infection and bacterial
pneumonia. International Journal of
tuberculosis and lung disease. 2001;
1:15. |
| 23. |
Kong
X T, Fang HT, Jiang G Q. treatment of
acute bronchiolitis with Chinese herbs.
Archives of diseases of childhood.1993;68:468-471. |
| 24. |
ARI
News letter. Cigarette smokers a risk
factor. Severing the national ARI control
program of Egypt. Issue No. 5:1985 ;
3-3. |
| 25. |
 2003.
2003. |
| 26. |
Williams
B G, Gouws E, Boschi P C, et al. infections
disease: Estimate of world wide distribution
of child deaths from acute respiratory
infections.2. Philadelphia, international
publication ; 2002 :25-32. |
| 27. |
Pandy
M R, Boleij J S, Smith K R. Indoor air
pollution in developing countries and
acute respiratory infections in children-
25.Singapora, Tropical medical publication
; 1989:327-428. |
| 28. |
Bedoya
VI aged V, Trujillo H. frequency of
RSV in hospitalized children with lower
respiratory tract infections in Colombia,
pediatric infections disease Journal.
1996; 15:1123-1124. |
| 29. |
Riley
I. Etiology of acute respiratory infections
in children in developing countries.
In: Douglas R M, Kirdy Eaton E, eds.
Acute respiratory infection in childhood.
34. Sydney, International workshop publication;
1985:33-41. |
| 30. |
Murray
C J L, Lopez A D. Global comparative
assessments in the health sector: disease
burden, expenditures and interventions
packages. Genera, world health organization;
1994:230-241. |
| 31. |
Schwartz
B. potential intervention for the prevention
of childhood pneumonia in developing
countries: the etiology and epidemiology
of acute lower respiratory infections
among young children in developing countries.
Pediatric infections disease Journal.
1995 ; 6:73-82. |
| 32. |
Al-
Jassar N F J. clinic- epidemiological
study of acute respiratory infections
(ARI) in children under 5 years of age.
A thesis submitted to the college of
medicine and the committee of post graduate
studies of AL-Mustansiriya University
of the requirements for the degree of
Master of Science in community medicine.
The Iraqi Journal of medical science.
1994; 10:200-207. |
| 33. |
Singh
M P, Nayar S I. Magnitude of acute respiratory
infections in less than five year's
children. Journal of community disease.
1996 ; 28:273-278. |
| 34. |
UNICEF\
WHO statement program for the control
of acute respiratory infections in children
in developing countries. 1986. |
| 35. |
Prober
C G. Infections disease. In :Behrman
Richard E, Kliegman Robert M, Jenson
Hal B, eds. Nelson textbook of pediatrics.
16th ed. China, W B Saunders Company;
2000:736-1100. |
| 36. |
Wright
P. Infections diseases. In: Behrman
Richard E, Kliegman Robert M, Jonson
Hal B, eds. Nelson Textbook of pediatrics.
16th ed. China, W B Saunders company;
2000: 736-1100. |
| 37. |
Yakio
I. common cold and secondary bacterial
infection. Asian medical Journal. 1989;
6:32-36. |
| 38. |
WHO\
ARI. Out patient management of young
children with ARI: program for the control
of acute respiratory infections. WHO:
Geneva. (1994). |
| 39. |
Rigotti
N A. respiratory problems in : Goroll
Allan H, May Lawrence A, Mulley Albert
G. Primary care medicine. 16. Oxford,
Black well scientific publication; 1987:
201-208. |
| 40. |
Al-
Fekaiki, Epidemiology of acute respiratory
tract in Iraq. Iraqi medical Journal.
1986; 34:65-73. |
| 41. |
Reilly
S, V revels R, Metsing M. clinical signs
of pneumonia in children attending ahospital
out patient department in Lesotho. 72.
Geneva, world health organization publication;
1994:113-118. |
| 42. |
Welliver
J, and Wellver R. Bronchiolities. Pediatric
in review journal. 1993; 14:134-139. |
| 43. |
Mc
Milan J A, Tristan D A, Weiner L B,
Prediction of the duration of hospitalization
in patients with respiratory syncytial
virus infection. In: Paulk. Pediatrics.
4th. New York, A wiley medical publication;
1988:22-26. |
| 44. |
Knudtson
M. Differential Diagnosis of pharyngitis
in children. Journal of pediatric health
care. 1994; 12: 320-323. |
| 45. |
Virgi
M. Otitis Media. In: Poal H, Pio A,
Glezen P, eds. Pediatric. 4ed. 14. New
York, AWiely medical publication; 1993:
320-323. |
| 46. |
Tronendle
J, Denimler G, Glezzen P, et al. Fatal
influenza B virus pneumonia in pediatric
patients. Pediatric infections disease
Journal . 1992; 11:117-119. |
| 47. |
UNICEF/
WHO statement 1986. Basic principles
for control of acute respiratory infections
in children in developing countries.
A joint UNICEF/ WHO statement. 1986. |
| 48. |
WHO\
ARI. Acute respiratory infections in
children : which are the factors ? (1990.e). |
| 49. |
Hays
E B, Hurwitz E S, Schonberger L B, et
al. RSV outbreak on American Samoa :
Evaluation of risk factors. American
Journal of diseases of children. 1989
; 143: 316-321. |
| 50. |
Tupasi
T E, Velmonte M A, sanvictores M E G,
et al. Determinants of morbidity and
mortality due to acute respiratory infections
: Implications for intervention. The
Journal of infections diseases. 1988;
157:615-623. |
| 51. |
Hall
C B. Respiratory syncytial Virus in:
Feigin R D, Cherry J D, eds. Text book
of pediatric infections diseases. 3rd
ed. Philadelphia, W B Saunders Company;
1992: 1633-1656. |
| 52. |
Mc
Millan J A, Tristan D A, Weiner L B,
eds. Prediction of the duration of hospitalization
in patients with RSV infection. In:
pediatrics. Higgins A P, sandstorm C,
Brandon R. 4th ed. Vol. 81. |
| 53. |
Qasim
N A, Al- Jassar N F. Risk factors of
acute respiratory infection(ARI) in
children under 5 years of age. Iraqi
Journal of community medicine. 1996;
9:109-116. |
| 54. |
Al-Karaguily
T S. Risk factors of pneumonia in children
under 6 years of age. Journal of Saddam
University. 1998; 2:103-111. |
| 55. |
Al-
Humairy E H. Risk factors of severe
pneumonia in children. A thesis submitted
to the Iraqi committee for medical specialization
in partial fulfillment of the requirement
for the degree of fellowship of Iraqi
committee for medical specialization-
pediatrics. The Iraqi Journal of medical
sciences. 1998; 4:20-25. |
| 56. |
Al-
Azzawi HK, Saadallah s, Mukhlis F A
, et al. bacterial etiology of acute
lower respiratory tract infections in
young Iraqi children. The Iraqi Journal
of Medical Science. 2003; 3:314-319. |
| 57. |
Al-
Shahabi S J, Niazi A D, Al-Obeidy A.
the role of passive smoking in children
with lower respiratory disease: a case
control study. The Iraqi Journal of
medical science. 2001; 1:306-311. |
| 58. |
Lob-
Levyt J. prevention of pneumonia in
children. Lancet. 1993; 341:821-822. |
| 59. |
Community
- Acquired. Respiratory infections in
children, December. 1996. retrieved
3 February, 2005. from http : //www.
Primary case; clinics in office practice.
Volume 23. No.4./ W.B, Saunders Company. |
| 60. |
Jamil
N F. the use of clinical features and
chest X-ray for the diagnosis of pneumonia
in children. Iraqi Journal of community
medicine. 2002; 15:22-25. |
| 61. |
ICCARI.
Childhood pneumonia : strategies to
meet the challenge. Bulletin of the
world health organization. 1991:52-56. |
| 62. |
Saddam
S H, Al-Tawil N G. factors associated
with severe pneumonia among children
under five years of age: a hospital
based case- control study. The Iraqi
Journal of medical science. 2002 : 2
:50-56. |
| 63. |
Zhang
Z J G, Gao L, Wang Z, et al. Acute respiratory
infections in childhood in Beijin:Epidemiological
studies at Dong Guan Brigade. Sydney,
International work shop publication
;1985 65-69. |
| 64. |
Ali
S. study of the prevalence of acute
respiratory tract infections and the
value of some laboratory test in the
diagnosis of lower respiratory tract
infections. International Journal of
epidemiology. 1989 ; 6:102-111. |
| 65. |
Cushing
A H, Samet J M, Lambert W E, et al .
Breast feeding reduces risk of respiratory
illness in infants. American Journal
of epidemiology. 1998 ; 147:203-209. |
| 66. |
Pickering
L K. Recommendations for care of children
in special circumstances in: Weiss I
P. Red Book : report of the committee
on infections disease. 25th ed. USA,
American Academy of pediatrics ; 2000
:83-159. |
| 67. |
Perera
B J, Ganesan S, Jayarasa J. the impact
of breast feeding practices on respiratory
and diarrheal disease in infancy. Journal
of tropical pediatric. 1999; 45:115-118. |
| 68. |
Nilay
E, velipasaglu S, Aktekin M. Incidence
of acute respiratory infection and the
relationship with some factors in infancy
in Antalya 44. Turkey, International
publication ; 2002:64-69. |
| 69. |
Azize
HB, Zulkifli HI, Kasim MS. Protective
and risk factors for acute respiratory
infection in hospitalized urban Malaysian
children : a case control study. Tropical
medical public health Jaurnal. 1995;
26: 280-285. |
| 70. |
Shah
N, Ramanakutty V, premile P G, Sathy
N. Risk factors for severe pneumonia
in children in south Kerala: a hospital
based case- control study. Journal of
tropical pediatric.1994; 40: 201-206. |
| 71. |
Victora
C G. Risk factors for deaths due to
respiratory infections among Brazilian
infants. International Journal of Epidemiology.
1989 ; 918-925. |
| 72. |
Aung
T, Tun M, Thein A. Knowledge, attitude
and practice of mothers in childhood
acute respiratory infection (ARI) in
southern Asian. Tropical medical public
health journal. 1994; 25:590-593. |
| 73. |
Yne
Chen M D, Shunzhang Yn, Wanxian Li.
Artificial feeding and hospitalization
in the first 18 months of life. Pediatrics
Journal. 1988; 81:231-245. |
| 74. |
Price
John F, and Greally pater. Infant feeding
wheezing and allergy : a prospective
study .Archives of disease in childhood
journal. 1993 :68:724-728 |
| 75. |
Taylor
B, Wadsworth J, Golding J, Butter N.
Breast feeding, bronchitis, and admission
for lower respiratory illness and gastro-
enteritis during the first five years.
Lancet. 1982 ; 1: 1227-1229. |
|
|
| 76. |
Chan
K N, Noble J C M, Elliman A, et al.
Airway responsiveness in Low Birth weight
children and their mothers. In: Dutta
K, Lodha R, Singhali et al. Archives
of disease in children. 63.Tokyo, International
puplication ; 1988:905-910. |
| 77. |
Jamison
D. Disease control priorities in developing
countries. New York, Oxford University
press ; 1993:96-101. |
| 78. |
Tupasi
T E. Nutritional and acute respiratory
infection. In : Douglas M R, Kirby Eaton
E, eds. Acute respiratory infection
in childhood. Sydney,International workshop
publication;1985:68-71. |
| 79. |
Hamid
M, Qazi S A, Khan M A. clinical, nutritional
and radiological features of pneumonia.
JAMA Journal.1979;46:95-99. |
| 80. |
Falad
A G, Tschppeler H, Green Wood B M, et
al. use of simple clinical signs to
predict pneumonia in young Gambia children
: the influence of malnutrition. Bulletin
of WHO. 1995 ; 73:299-304. |
| 81. |
Rahman
M M, Rahman A M F. Prevalence of acute
respiratory tract infection and its
risk factors in under five children.
Bangladesh medical research council
Buletin, 1997 ; 23:47-50. |
| 82. |
Rice
A L, Sacco L, Adnan H, Black R E. Malnutrition
as an underlying cause of childhood
deaths associated with infections diseases
in developing countries. Bulletin of
the world health organization . 2000
; 78:1207-1220. |
| 83. |
Defrancisco
A. Risk factors for mortality from acute
lower respiratory tract infections in
young Gambian children. International
Journal of Epidemiology. 1993 ; 22:1174-1182. |
| 84. |
Yoon
P W. the effect of malnutrition on the
risk of diarrhea and respiratory mortality
in children less than 2 year of age
in Cebu, Philippines. American Journal
of clinical nutrition. 1997 ; 65:1070-1077. |
| 85. |
Berman
S. Denans A. Bedoya A, Constain V, sag
Berrero I. Acute lower respiratory tract
illnesses in Cali : A two -year ambulatory
study . Pediatric Journal . 1983 ; 71:
210-218. |
| 86. |
WHO.
World health report , life in 21st century.
Aviation for all. WHO\ Geneva. (1998). |
| 87. |
Broor
S, Pandey R M, Ghosh M, et al. Risk
factors for severe acute lower respiratory
tract infection in under five years
children. 38. India, university of Neodelhi
; 2001 :1361-1369. |
| 88. |
Pio
A.Acute respiratory infection in children
in developing countries:an international
point of view pediatric infection diseases.Geneva,WHO
publication;1986:179-181. |
| 89. |
Beneson
A S. control of communicable diseases
in man : An official report of the American
public health association. 14th ed.
USA, John and Lucas publication ; 1985:233-258. |
| 90. |
ARI
News- News and reviews of acute respiratory
infections and diarrheal disease. Issue
3.1985. |
| 91. |
Rshid
SF, Hadi A, Afsana K, Begum S. Acute
respiratory infections in rural Bangladesh
: Cultural understandings, practices
and the role of mothers and community
health volunteers. Tropical medical
public health journal. 2001 ; 6:249-255. |
| 92. |
Mc
Connchie K M, Roghmann. Parental smoking,
presence of older siblings and family
history of asthma. Increase risk of
bronchiolitis. American Journal of disease
of children. 1986 ; 140: 806-812. |
| 93. |
World
Resources Institute, UNEP, UNDP, World
Band, 1998-1999- World Resources : a
guide to the global environment. Oxford,
Oxford University press. 1998. |
| 94. |
Engel
P, Hurtado E, Ruel M. smoke exposure
of women and young children in highland
Guatemala: predictions and recall accuracy.
54. Guatemala, publication of Human
organization ; 1998 :408-417. |
| 95. |
Norboo
T, Angchuk P T. Silicosis in a Himalayan
village population. In : Tahya M. Role
of environmental dust. 3rd ed .46. Amsterdam,
north Holland publishing company ; 1991:
341-343. |
| 96. |
Behera
D, Dash S, Malik S-Blood carboxy- hemoglobin
Levels following a cute exposure to
smoke of biomass fuel. Indian Journal
of medical Reseasrch.1998 ; 88:522-542. |
| 97. |
Kossove
D. smoke-filled rooms and lower respiratory
diseases in infants. South African medical
Journal. 1982 ; 61:622-524. |
| 98. |
Colling
D A, Sithole S D, Martin S K. Indoor
wood smoke pollution causing lower respiratory
disease in children. Journal of tropical
pediatric. 1990 ;20:151-155. |
| 99. |
Morris
K. Wood-burning stoves and lower respiratory
tract infection in American Indian .
American Journal of diseases of children.
1990 ; 144:105-108. |
| 100. |
Robin
L F . Wood-burning stoves and lower
respiratory illnesses in Navajo children
pediatric infections diseases Journal.
1996 ; 15:859-865. |
| 101. |
Strachan
D P, cook D G. parental smoking , middle
ear disease and adenotosillectomy in
children. Pediatric infections disease
Journal. 1998 ; 53: 50-56. |
| 102. |
Orenstein
D M. the respiratory system. In : Beherman
Richard E, Kliegman M Robert, Jenson
Hal B, eds. Nelson textbook of pediatrics.
16th ed. China. W B saunders company
; 2000: 1235-1236. |
| 103. |
Simes
D G, Downham M A, Mc Qillan J, et al.
RSV infection in north east England.
British medical Journal . 1976 ; 2:1095. |
| 104. |
WHO\
ARI. Program of control of cute respiratory
infections. Interim program report.
1991. |
| 105. |
Ratman
S, West R, Gadag V. Measles and rubella
antibody response after measles, mumps,
rubella vaccination in children with
febrile upper respiratory tract infection.
Journal of pediatric. 1995 ; 127: 432-434. |
| 106. |
Miller
D L. Acute respiratory infections :
short term interventions and long term
strategy. World health organization
chronicle. 1978 ; 32: 286-290. |
| 107. |
Biswas
A, Biswas R, Manna B, et al. Risk factors
of acute respiratory infections in under
5 of urban slum community. Indian public
health Journal. 1999 ; 43:73-75. |
| 108. |
Fergusson
D m, Horwood L J, Shannon F T, et al.
parental smoking and lower respiratory
illness in the first three years of
life. Journal of Epidemiology and community
health. 1981 ; 35:180-184. |
| 109. |
ARI
News letter. Issue No. 28 April- July.
1994. |
| 110. |
Lopez
B I M, Sepulveda H, Valdes I. Acute
respiratory illnesses in the first 18
months of life. The Journal of pediatric.
1997 ; 1:9-17. |
| 111. |
Weitzman
M, Gortmaker S, Walker D, Sobel A. maternal
smoking and childhood asthma. Pediatric
journal. 1990 ; 85:5050-511. |
| 112. |
Fleming
D W, Cochi S L, High tower A W, et al.
childhood upper respiratory tract infections:
to what degree its affected by day-care
attendance. The Journal of pediatric.
1989 ; 3: 266-273. |
| 113. |
Diseases
transmitted by respiratory infection.
2001. retrieved 7, April, 2005. from
http : // www. Google. Internet / MBI.
Microorganisms and human disease / respiratory
infection study guide. |
| 114. |
Ware
J H, Dockery D W, Spiro A, et al. American
review of respiratory disease : passive
smoking and respiratory health of children
living in six cities. 3. USA, A Wiley
medical publication ; 1984: 366-374. |
| 115. |
Pedreira
F A, Guandolo V L, feroli E J. Involuntary
smoking and incidence of respiratory
illness during the first year of life.
In: Mella G M, Weiss I P, eds. Pediatrics.
3rd ed. 75. New York, A Wiely medical
publication. 1985: 594-597. |
| 116. |
Mclntosh
K. infections disease. In :Beherman
R E, Kligman R M, Jenseon H, eds.Nelson
textbook of pediatrics. 16th ed. China,
W B Saunders company ; 2000 :736-1100. |
| 117. |
Avila
MM, Carballal G, Rovaletti H, Ebekian
B, Cusminsky M. Viral etiology of acute
lower respiratory infection in children
from closed community. American review
respiratory disease. 1989 ; 140:634-637. |
| 118. |
Kjolhada-Cl,
Chew-FJ, Godo A. clinical trial of vitamin
A as adjustant treatment for lower respiratory
tract infections. Pediatric journal.
1995:126:807-812. |
| 119. |
Respiratory
syncytial virus infection in children
under one year of age hospitalized for
acute respiratory diseases in pelotas,
RS. Journal de pneumologia vol. 29/no.1
Sao Paulo Jan./ Feb.2003/ PDF format.
Retrieved 13 Fabruary, 2005, from http
: // www. Seielo Brazil. |
| 120. |
Macedo
C G. Infant mortality in Americans.
Bulletin of the pan American health
organization. 1998 ; 22:3. |
| 121. |
Yakio
inuyama. Common cold and secondary bacterial
infection. Asian medical Journal . 1989
: 32-36. |
| 122. |
Katcher
M L. cold, cough and allergy medications
: uses and abuses. In Anderson L J.
pediatric in review 17. Amsterdam, north
Holland publishing company ; 1996: 1-44. |
| 123. |
Gephart
H R. Minor acute illnesses of childhood
in primary care. Journal of medical
research. 1988 ; 3:409-418. |
| 124. |
Mickenzie
S, Silverman M. respiratory disorders.
In : forfor K, Arenil R. forfor and
Arenil's textbook of pediatrics. 5th
ed. Edinburgh, Churchill living stone
; 1998 : 489-583. |
| 125. |
Mohammed
T A. knowledge, attitude and practice
of mothers regarding acute respiratory
infections in children. A study submitted
to medical college - Saddam university
of the requirement for the degree of
Master Science in community medicine.
1995 ; 10:334-339. |
| 126. |
Viral
respiratory infections. April, 1998
; 53 : 302-307. retrieved 3 Jan, 2005,
from http : // www. Thorax / Lung infections
bullet. |
| 127. |
Frank
H S, Kate H, David T. Acute lower respiratory
tract infections in children: possible
criteria for selection of patients for
antibiotic therapy and hospital admission.
Bulletin of the world health organization.
2003 ; 81:301-305. |
| 128. |
WHO\
ARI. Acute respiratory infections in
children : case management in small
hospital in developing countries. A
manual for doctors and other senior
health workers. (1990.d). |
| 129. |
Pio
A, Leowski J, Ten D H G. the magnitude
of the problem of acute respiratory
infections. Sydney, International workshop
publication ; 1985:3-16. |
| 130. |
Mulholl
and E K, olinsky A, Shann A F. clinical
practice, clinical findings and severity
of acute bronchiolitic. 335. Oxford.
International publication ; 1990 :1259-1261. |
| 131. |
Mansell
A L, Driscoll J M, James L S. pulmonary
follow up of moderately low birth weight
in infant with and without respiratory
distress syndrome. The Journal of pediatrics.
1987 ; 100:111-115. |
| 132. |
Respiratory
tract infections fact sheet. 2004. retrieved
12 March, 2005,from http : //www. Google.
Internet / Nebraska Health and human
services system. |
| 133. |
Hamill
P V, Drizd T A, Johnson C L, et al.
physical growth: National center for
health statistics percentiles. The American
Journal of clinical Nutrition. 1979
; 32:607-629. |
| 134. |
Schidlow
D V, Callahan C W. pneumonia in : Anderson
L J. pediatric in review. 17 Amsterdam,
north Holland publicating company ;
1996: 299-331. |
| 135. |
Appropriate
use of antibiotics for URIs in children
: part II. Cough, pharyngitis and the
common cold. / AAFP. October 15, (1998),
retrieved 22. March, 2005, from http
: // www. American family physician.
Internet / AAFP, Journal, vol 58 / No.
6. |
| 136. |
Shann
F, Hart K, Thomas D. Acute lower respiratory
tract infections in children : possible
criteria for selection of patient for
antibiotics therapy and hospital admission.
Bulletin of the world health organization.
1984 ; 62: 749-753. |
| 137. |
Cunha
A, Jose L, Alves d. Management of acute
respiratory infections in children evaluation
in Rio de Janeiro health care facilities.
Rio de Janeiro, cadsaude publication
; 2002:55-610. |
| 138. |
Pichichero
M E, Green J L, Francis A B. outcomes
after Judicious antibiotics used for
respiratory tract infection seen in
a private pediatric. Journal of medical
research. 2000 ; 105:753-759. |
| 139. |
Persons
C, Shann F. The standardized clinical
management of acute respiratory infections
in children in developing countries.
In : Douglas R M Kirby, Eaton E, eds.
Acute respiratory infections in childhood.
Sydney , proceeding of an international
workshop ; 1985:185-186. |
| 140. |
Patient
information : The acute respiratory
tract infections. 2001. retrieved 11
November, 2004 : from http : //www.
Up to date patient information / ASN
/ SGIM, society of General International
medicine. |
| 141. |
Gve
S. Remedies for young children. ARI
news. 1990 ; 18:2-3. |
| 142. |
Ramussen
F. and smodby B. life lable methods
applied to use of medical care and prescription
drugs in early childhood. Journal of
epidemiology and community health ,
1989 ; 43: 140-146. |
| 143. |
Herman
I, Elsa D, Giron M, et al. Epidemiology
of acute respiratory infections in pre
school children of rural Guatemala.
22. USA, Bulletin of the pan American
health organization ; 1988 : 383-393. |
| 144. |
Tupasi
T E. Malnutrition and acute respiratory
infections in philppino children. In:
ferris B G. reviews of infections disease.
12. Philppino, Tropical medical publication
; 1990: 1047-1054. |
| 145. |
Al-
Tae'e N K. Attitude and practice of
mothers in Al-Sheikh omar health center
regarding acute respiratory infection
of children. A dissertation submitted
to the scientific council of community
medicine in partial fulfillment for
the degree for fellowship of the Iraqi
commission for medical specialization
in community medicine. Iraqi Journal
of community medicine. 1998 ; 4:27-31. |
| 146. |
Al-
Bargish K L, Hasony H J . Respiratory
syncytial virus infection young children
with acute respiratory tract infection
in Iraq. Eastern Mediterranean Health
Journal. 1999 ; 5:941-947. |
| 147. |
Fonseca
W, Kekwood BR, Victoria CJ, et al. Risk
factors for children pneumonia among
the Urban poor in Fortaleza : Brazil
a case - control studt. Bulletin of
the world health organization. 1996
; 74 : 199-208. |
| 148. |
Rashid
S F, Hadi A, Afsana K,Begum S A. acute
respiratory infections in rural Bangladesh
: cultural understandings, practices
and the role of mothers and community
health volunteers. Tropical medical
public health Journal . 2001 ; 6 : 249-255. |
| 149. |
Aung
T, Tun KM, Thein A A. knowledge, attitude
and practice of mothers on childhood
acute respiratory infection (ARI) in
southern Asian. Topical medical public
health Journal . 1994 ; 25 : 590-593. |
| 150. |
Respiratory
Viral Diseases. The merck Manual, sec.
13. ch. 162, viral diseases. 2001. Retrieved
8 January, 2005, from http ://www. Google.
Internet/ the merck manual of diagnosis
and therapy. |
|
| |
|
|
|
|